
Chemistry: An Introduction to General, Organic, and Biological Chemistry, Books a la Carte Edition (13th Edition)
13th Edition
ISBN: 9780134554631
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.53UTC
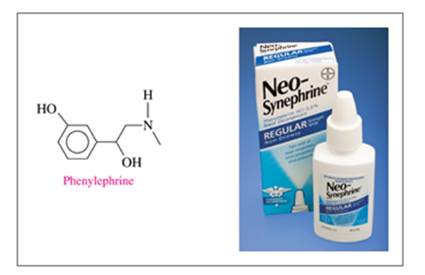
Phenylephrine is the active ingredient in some nose sprays used to reduce swelling of nasal membranes. Identify the
(14.1, 14.3, 14.5, 14.6)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(28 pts.) 7. Propose a synthesis for each of the following transformations. You must include the
reagents and product(s) for each step to receive full credit. The number of steps is provided.
(OC 4)
4 steps
4 steps
OH
b.
LTS
Solid:
AT=Te-Ti
Trial 1
Trial 2
Trial 3
Average
ΔΗ
Mass water, g
24.096
23.976
23.975
Moles of solid, mol
0.01763
001767
0101781
Temp. change, °C
2.9°C
11700
2.0°C
Heat of reaction, J
-292.37J -170.473
-193.26J
AH, kJ/mole
16.58K 9.647 kJ 10.85 kr
16.58K59.64701
KJ
mol
12.35k
Minimum AS,
J/mol K
41.582
mol-k
Remember: q = mCsAT (m = mass of water, Cs=4.184J/g°C) & qsin =-qrxn &
Show your calculations for:
AH in J and then in kJ/mole for Trial 1:
qa (24.0969)(4.1845/g) (-2.9°C)=-292.37J
qsin =
qrxn =
292.35 292.37J
AH in J = 292.375 0.2923kJ
0.01763m01
=1.65×107
AH in kJ/mol =
=
16.58K
0.01763mol
mol
qrx
Minimum AS in J/mol K (Hint: use the average initial temperature of the three trials, con
Kelvin.)
AS=AHIT
(1.65×10(9.64×103) + (1.0
Jimai
For the compound: C8H17NO2
Use the following information to come up with a plausible structure:
8
This compound has "carboxylic acid amide" and ether functional groups.
The peaks at 1.2ppm are two signals that are overlapping one another.
One of the two signals is a doublet that represents 6 hydrogens; the
other signal is a quartet that represents 3 hydrogens.
Chapter 14 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry, Books a la Carte Edition (13th Edition)
Ch. 14.1 - What carboxylic acid is responsible for the pain...Ch. 14.1 - What carboxylic acid is found in vinegar?Ch. 14.1 - Prob. 14.3PPCh. 14.1 - Prob. 14.4PPCh. 14.1 - Draw the condensed structural formulas for a and b...Ch. 14.1 - Draw the condensed structural formulas for a and b...Ch. 14.2 - Identify the compound in each group that is most...Ch. 14.2 - Prob. 14.8PPCh. 14.2 - Prob. 14.9PPCh. 14.2 - Prob. 14.10PP
Ch. 14.2 - Prob. 14.11PPCh. 14.2 - Prob. 14.12PPCh. 14.2 - Prob. 14.13PPCh. 14.2 - Prob. 14.14PPCh. 14.3 - Prob. 14.15PPCh. 14.3 - Prob. 14.16PPCh. 14.3 - Prob. 14.17PPCh. 14.3 - Prob. 14.18PPCh. 14.3 - Prob. 14.19PPCh. 14.3 - Prob. 14.20PPCh. 14.3 - Prob. 14.21PPCh. 14.3 - Prob. 14.22PPCh. 14.3 - Prob. 14.23PPCh. 14.3 - Prob. 14.24PPCh. 14.4 - What are the products of the acid hydrolysis of an...Ch. 14.4 - Prob. 14.26PPCh. 14.4 - Prob. 14.27PPCh. 14.4 - Prob. 14.28PPCh. 14.5 - Prob. 14.29PPCh. 14.5 - Prob. 14.30PPCh. 14.5 - Prob. 14.31PPCh. 14.5 - Prob. 14.32PPCh. 14.5 - Prob. 14.33PPCh. 14.5 - Prob. 14.34PPCh. 14.5 - Prob. 14.35PPCh. 14.5 - Prob. 14.36PPCh. 14.5 - Prob. 14.37PPCh. 14.5 - Prob. 14.38PPCh. 14.6 - Prob. 14.39PPCh. 14.6 - Prob. 14.40PPCh. 14.6 - Prob. 14.41PPCh. 14.6 - Prob. 14.42PPCh. 14.6 - Prob. 14.43PPCh. 14.6 - Prob. 14.44PPCh. 14.6 - Draw the condensed structural or line-angle...Ch. 14.6 - Draw the condensed structural or line-angle...Ch. 14.6 - a. Identify the functional groups in dicyclanil....Ch. 14.6 - a. Identify the functional groups in enrofloxacin....Ch. 14 - Prob. 14.49UTCCh. 14 - Prob. 14.50UTCCh. 14 - The ester methyl butanoate has the odor and flavor...Ch. 14 - Prob. 14.52UTCCh. 14 - Phenylephrine is the active ingredient in some...Ch. 14 - Melatonin is a naturally occurring compound in...Ch. 14 - Prob. 14.55UTCCh. 14 - Prob. 14.56UTCCh. 14 - Prob. 14.57APPCh. 14 - 14.58 Write the IUPAC and common names, if any,...Ch. 14 - Prob. 14.59APPCh. 14 - Prob. 14.60APPCh. 14 - Draw the condensed structural or line-angle...Ch. 14 - Prob. 14.62APPCh. 14 - Prob. 14.63APPCh. 14 - 14.64 Draw the condensed structural or line-angle...Ch. 14 - Prob. 14.65APPCh. 14 - 14.66 Write the common name and classify each of...Ch. 14 - Prob. 14.67APPCh. 14 - Draw the condensed structural or line-angle...Ch. 14 - Prob. 14.69APPCh. 14 - Prob. 14.70APPCh. 14 - Write the IUPAC name for each of the following:...Ch. 14 - Prob. 14.72APPCh. 14 - Prob. 14.73APPCh. 14 - Prob. 14.74APPCh. 14 - Prob. 14.75APPCh. 14 - Toradol is used in dentistry to relieve pain....Ch. 14 - Prob. 14.77CPCh. 14 - Draw the line-angle formula and write the IUPAC...Ch. 14 - Prob. 14.79CPCh. 14 - Prob. 14.80CPCh. 14 - Prob. 14.81CPCh. 14 - Prob. 14.82CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Vnk the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest bolling point, choose 2 next to the substance with the next highest boiling point, and so on. substance C D chemical symbol, chemical formula or Lewis structure. CH,-N-CH, CH, H H 10: H C-C-H H H H Cale H 10: H-C-C-N-CH, Bri CH, boiling point (C) Сен (C) B (Choosearrow_forwardPlease help me find the 1/Time, Log [I^-] Log [S2O8^2-], Log(time) on the data table. With calculation steps. And the average for runs 1a-1b. Please help me thanks in advance. Will up vote!arrow_forwardQ1: Answer the questions for the reaction below: ..!! Br OH a) Predict the product(s) of the reaction. b) Is the substrate optically active? Are the product(s) optically active as a mix? c) Draw the curved arrow mechanism for the reaction. d) What happens to the SN1 reaction rate in each of these instances: 1. Change the substrate to Br "CI 2. Change the substrate to 3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF 4. Increase the substrate concentration by 3-fold.arrow_forward
- Experiment 27 hates & Mechanisms of Reations Method I visual Clock Reaction A. Concentration effects on reaction Rates Iodine Run [I] mol/L [S₂082] | Time mo/L (SCC) 0.04 54.7 Log 1/ Time Temp Log [ ] 13,20] (time) / [I] 199 20.06 23.0 30.04 0.04 0.04 80.0 22.8 45 40.02 0.04 79.0 21.6 50.08 0.03 51.0 22.4 60-080-02 95.0 23.4 7 0.08 0-01 1970 23.4 8 0.08 0.04 16.1 22.6arrow_forward(15 pts) Consider the molecule B2H6. Generate a molecular orbital diagram but this time using a different approach that draws on your knowledge and ability to put concepts together. First use VSEPR or some other method to make sure you know the ground state structure of the molecule. Next, generate an MO diagram for BH2. Sketch the highest occupied and lowest unoccupied MOs of the BH2 fragment. These are called frontier orbitals. Now use these frontier orbitals as your basis set for producing LGO's for B2H6. Since the BH2 frontier orbitals become the LGOS, you will have to think about what is in the middle of the molecule and treat its basis as well. Do you arrive at the same qualitative MO diagram as is discussed in the book? Sketch the new highest occupied and lowest unoccupied MOs for the molecule (B2H6).arrow_forwardQ8: Propose an efficient synthesis of cyclopentene from cyclopentane.arrow_forward
- Q7: Use compound A-D, design two different ways to synthesize E. Which way is preferred? Please explain. CH3I ONa NaOCH 3 A B C D E OCH3arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward(10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forward
- Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardQ3: Rank the following compounds in increasing reactivity of E1 and E2 eliminations, respectively. Br ca. go do A CI CI B C CI Darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry In Focus
Chemistry
ISBN:9781305084476
Author:Tro, Nivaldo J., Neu, Don.
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY