
The dissociation of molecular iodine into iodine atoms is represented as
At 1000 K, the equilibrium constant Kc for the reaction is 3.80 × 10−5. Suppose you start with 0.0456 mole of I2 in a 2.30-L flask at 1000 K. What are the concentrations of the gases at equilibrium?
Interpretation:
The equilibrium concentration of hydrogen and iodine gas has to be calculated.
Concept Introduction:
Equilibrium concentration: If Kc and the initial concentration for a reaction and calculate for both equilibrium concentration, and using the (ICE) chart and equilibrium constant and derived changes in respective reactants and products.
Equilibrium constant: Concentration of the products to the respective molar concentration of reactants it is called equilibrium constant. If the K value is less than one the reaction will move to the left side and the K values is higher (or) greater than one the reaction will move to the right side of reaction.
Heterogeneous equilibrium: This equilibrium reaction does not depend on the amounts of pure solid and liquid present, in other words heterogeneous equilibrium, substances are in different phases.
Kp and Kc: This equilibrium constants of gaseous mixtures, these difference between the two constants is that Kc is defined by molar concentrations, whereas Kp is defined by the partial pressures of the gasses inside a closed system.
Vaporized equilibrium: This conversion of liquid in gaseous phase is known as vaporization process. At starting the rate of condensation is less than the rate of evaporation but as evaporation continues the concentration of gaseous molecule in the vapour phase increase.
Answer to Problem 14.44QP
Explanation of Solution
To find: The each reactant product equilibrium concentration should be identified given the gas phase reaction.
Write and Analyze the given gas phase chemical equilibrium reaction.
The given equilibrium reaction has a homogenous process, then the equilibrium constant can also be represented by Kp, were the Kp represents partial pressure. Then the product molecule partial pressure 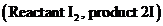 is derived in step-2.
is derived in step-2.
To find: Calculate equilibrium concentration (Kp) values for given the statement of equilibrium reaction.
Calculate and analyze the (Kp) values at
We derived here (Kp) values of (I2) dissociation reaction
First we derived the initial concentration of (I2) is
We consider the equilibrium expression in terms of the equilibrium concentration.
The obtained second (x) values are negative concentration, this physically impossible so we omitted this values. First (x) value is correct one.
The given iodine dissociation equilibrium reaction the respective reactant to give the two moles of products in the gas phase and this equilibrium reaction expression contains single conditions like gases phase, the equilibrium constant can also be represented by Kp, were the “P” partial pressure. The each molar concentration values are Kp derived given the gas phase reaction at
The molar concentration (M) values are derived given the iodine
Want to see more full solutions like this?
Chapter 14 Solutions
ALEKS 360; 18WKS F/ GEN. CHEMISTRY >I<
- Basic strength of organic bases.arrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor” *see attachedarrow_forward
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





