
Concept explainers
(a)
Interpretation:
Whether the given molecule is
Concept introduction:
The molecules, to be aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic due to lack of planarity.
Explanation of Solution
The given structure is:
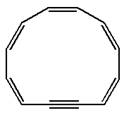
The molecule has total
The molecule is determined as nonaromatic based on the structure planarity.
(b)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic due to lack of planarity.
Explanation of Solution
The given structure is:
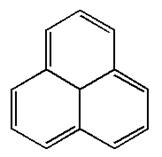
The molecule has total
The molecule is determined as nonaromatic based on the structure planarity.
(c)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is aromatic as it is planar, has cyclic conjugation, and obeys Hückel’s rule.
Explanation of Solution
The given structure is:
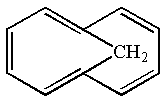
The given molecule is planar and has total
The molecule is determined as aromatic based on the Hückel’s rule.
(d)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic due non cyclic conjugated system.
Explanation of Solution
The given structure is:
![]()
The molecule has a conjugated system of total
The molecule is determined as nonaromatic based on the non cyclic structure which lacks cyclic conjugation.
(e)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is aromatic as it is planar, has cyclic conjugation, and obeys Hückel’s rule.
Explanation of Solution
The given structure is:
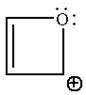
The molecule is planar and has fully cyclic conjugated system of total
The molecule is determined as aromatic based on planarity, fully conjugated system, and having Hückel’s rule of
(f)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic as it is not conjugated and does not obey Hückel’s rule.
Explanation of Solution
The given structure is:
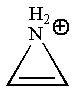
The molecule has only
The molecule is determined as nonaromatic based on the non-conjugated system of
(g)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic as it has no cyclic conjugated system.
Explanation of Solution
The given structure is:
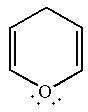
One of the lone pair on the oxygen atom participates in the resonance, thus, the molecule has
The molecule is determined as nonaromatic based on non-conjugated system of
Want to see more full solutions like this?
Chapter 14 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

