
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.28P
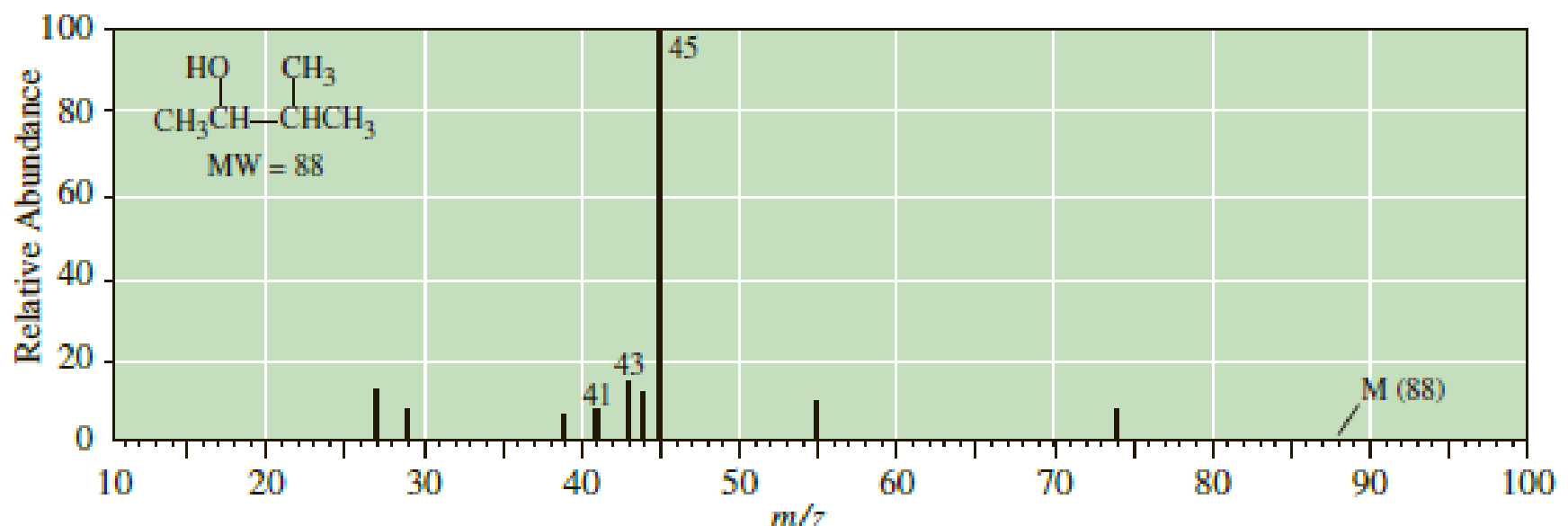
Following is the mass spectrum of 3-methyl-2-butanol. The molecular ion m/z 88 does not appear in this spectrum. Propose structural formulas for the cations of m/z 45, 43, and 41.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Using the critical constants for water
(refer to the table in the lecture slides),
calculate the second virial coefficient.
Assume that the compression factor (Z)
is expressed as an expansion series in
terms of pressure.
+3413 pts
/4800
Question 38 of 48
>
Write the full electron configuration for a Kion.
© Macmillan Learning
electron configuration:
↓
Resources
Solution
Penalized
→ Al Tutor
Write the full electron configuration for an Fion.
electron configuration:
T
G
6
&
7
Y
H
כ
Y
00
8
hp
9
J
K
no
L
144
P
112
|
t
KC
47°F Clear
ins
prt sc
delete
]
backspace
er
How to solve these types of problems step by step? I'm so confused.
Chapter 14 Solutions
Organic Chemistry
Ch. 14.2 - Calculate the nominal mass of each ion. Unless...Ch. 14.3 - Propose a structural formula for the cation at m/z...Ch. 14.3 - The low-resolution mass spectrum of 2-pentanol...Ch. 14 - Draw acceptable Lewis structures for the molecular...Ch. 14 - The molecular ion for compounds containing only C,...Ch. 14 - For which compounds containing a heteroatom (an...Ch. 14 - The so-called nitrogen rule states that if a...Ch. 14 - Prob. 14.8PCh. 14 - Prob. 14.9PCh. 14 - Prob. 14.10P
Ch. 14 - Determine the probability of the following in a...Ch. 14 - The molecular ions of both C5H10S and C6H14O...Ch. 14 - Prob. 14.13PCh. 14 - Carboxylic acids often give a strong fragment ion...Ch. 14 - For primary amines with no branching on the carbon...Ch. 14 - Prob. 14.16PCh. 14 - A characteristic peak in the mass spectrum of most...Ch. 14 - Predict the relative intensities of the M and M +...Ch. 14 - The mass spectrum of compound A shows the...Ch. 14 - The mass spectrum of compound B, a colorless...Ch. 14 - Write molecular formulas for the five possible...Ch. 14 - Write molecular formulas for the five possible...Ch. 14 - The molecular ion in the mass spectrum of...Ch. 14 - Prob. 14.24PCh. 14 - Following is the mass spectrum of 1-bromobutane....Ch. 14 - Following is the mass spectrum of...Ch. 14 - Following is the mass spectrum of an unknown...Ch. 14 - Following is the mass spectrum of...Ch. 14 - Prob. 14.29PCh. 14 - Following are mass spectra for the constitutional...Ch. 14 - 2-Methylpentanal and 4-methyl-2-pentanone are...Ch. 14 - Prob. 14.32PCh. 14 - Account for the presence of the following peaks in...Ch. 14 - All methyl esters of long-chain aliphatic acids...Ch. 14 - Propylbenzene, C6H5CH2CH2CH3, and isopropyl...Ch. 14 - Account for the formation of the base peaks in...Ch. 14 - Prob. 14.37PCh. 14 - Prob. 14.38P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give the IUPAC name for each compound.
Organic Chemistry
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the expected product of the following Claisen rearrangement. || = IV OV 00000 5 ОН Он Он Он Он || III IV Varrow_forwardCan you please color-code and explain how to solve this and any molecular orbital diagram given? I'm so confused; could you provide baby steps regardless of which problem type they gave me?arrow_forwardConsider the following structure. OH Esmolol The synthesis of this compound uses a building block derived from either ethylene oxide or epichlorohydrin. 1) Determine which building block was used: | 2) Draw the structure of the nucleophiles that were used along with this building block in the synthesis of the molecule. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. You do not have to consider stereochemistry. Θε {n [arrow_forward
- < 10:44 5GW 10 Question 7/8 Show Answer Convert 46.0 mm to inches (1 inch = 2.54 cm) 46.0 DAM STARTING AMOUNT 1 cm 1 in 46.0 mm x ☑ 10 mm 10 cm ADD FACTOR DELETE x() X × = 1.81 in = 1 10 Dam ANSWER RESET ១ 2.54 0.0460 mm 10 1000 in 0.001 11.7 m 4.60 18.1 cm 100 1.81 0.394 1 0.1 46.0 0.01 Tap here for additional resourcesarrow_forward< 10:44 Question 6/8 5GW (10 Submit A cake recipe calls for 230.0 mL of buttermilk. How 230.0 many cups is this? DAL STARTING AMOUNT × 1 cups 230.0 mL x = 0.9722 cups 230.0 mL ADD FACTOR DELETE (( ) = 1 cups 230.0 DAE ANSWER RESET ១ 9.722 × 105 0.8706 cups 8.706 × 104 1 L 8.706 × 105 0.9722 quart 10 100 mL 0.001 0.1 6.076 × 103 0.01 9.722 × 104 230.0 0.06076 4 1.0567 1000 6.076 × 104 Tap here for additional resourcesarrow_forward< 10:44 Question 6/8 5GW (10 Submit A cake recipe calls for 230.0 mL of buttermilk. How 230.0 many cups is this? DAL STARTING AMOUNT × 1 cups 230.0 mL x = 0.9722 cups 230.0 mL ADD FACTOR DELETE (( ) = 1 cups 230.0 DAE ANSWER RESET ១ 9.722 × 105 0.8706 cups 8.706 × 104 1 L 8.706 × 105 0.9722 quart 10 100 mL 0.001 0.1 6.076 × 103 0.01 9.722 × 104 230.0 0.06076 4 1.0567 1000 6.076 × 104 Tap here for additional resourcesarrow_forward
- Show work in detailed of all the options. Don't give Ai generated solutionarrow_forwardPredict the Product. Predict the major organic product for the following reaction:arrow_forwardPlease provide the complete mechanism for the reaction below including arrows, intermediates, and formal charges.arrow_forward
- Can you please explain this to me? Maybe color-code it in essence and highlight it.arrow_forwardCan you please color-code and explain this problem to me and is it because its spdf, and then it follows by higher numver so 3 first and so forth ...arrow_forwardapp aktv.com Alt Leaming App Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 30 of 35 Na Select to Edit Arrows THE M 回 Na :0: 0% Donearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY