
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14.2, Problem 14.1P
Calculate the nominal mass of each ion. Unless otherwise indicated, use the mass of the most abundant isotope of each element.
(a) 
(b) 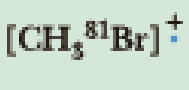
(c) 
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.
Write the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.
Name the following using IUPAC.
Chapter 14 Solutions
Organic Chemistry
Ch. 14.2 - Calculate the nominal mass of each ion. Unless...Ch. 14.3 - Propose a structural formula for the cation at m/z...Ch. 14.3 - The low-resolution mass spectrum of 2-pentanol...Ch. 14 - Draw acceptable Lewis structures for the molecular...Ch. 14 - The molecular ion for compounds containing only C,...Ch. 14 - For which compounds containing a heteroatom (an...Ch. 14 - The so-called nitrogen rule states that if a...Ch. 14 - Prob. 14.8PCh. 14 - Prob. 14.9PCh. 14 - Prob. 14.10P
Ch. 14 - Determine the probability of the following in a...Ch. 14 - The molecular ions of both C5H10S and C6H14O...Ch. 14 - Prob. 14.13PCh. 14 - Carboxylic acids often give a strong fragment ion...Ch. 14 - For primary amines with no branching on the carbon...Ch. 14 - Prob. 14.16PCh. 14 - A characteristic peak in the mass spectrum of most...Ch. 14 - Predict the relative intensities of the M and M +...Ch. 14 - The mass spectrum of compound A shows the...Ch. 14 - The mass spectrum of compound B, a colorless...Ch. 14 - Write molecular formulas for the five possible...Ch. 14 - Write molecular formulas for the five possible...Ch. 14 - The molecular ion in the mass spectrum of...Ch. 14 - Prob. 14.24PCh. 14 - Following is the mass spectrum of 1-bromobutane....Ch. 14 - Following is the mass spectrum of...Ch. 14 - Following is the mass spectrum of an unknown...Ch. 14 - Following is the mass spectrum of...Ch. 14 - Prob. 14.29PCh. 14 - Following are mass spectra for the constitutional...Ch. 14 - 2-Methylpentanal and 4-methyl-2-pentanone are...Ch. 14 - Prob. 14.32PCh. 14 - Account for the presence of the following peaks in...Ch. 14 - All methyl esters of long-chain aliphatic acids...Ch. 14 - Propylbenzene, C6H5CH2CH2CH3, and isopropyl...Ch. 14 - Account for the formation of the base peaks in...Ch. 14 - Prob. 14.37PCh. 14 - Prob. 14.38P
Additional Science Textbook Solutions
Find more solutions based on key concepts
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Give the IUPAC name for each compound.
Organic Chemistry
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Atomic Number, Atomic Mass, and the Atomic Structure | How to Pass ChemistryThe Nucleus: Crash Course Chemistry #1; Author: Crash Course;https://www.youtube.com/watch?v=FSyAehMdpyI;License: Standard YouTube License, CC-BY