
Each of the following alcohols is named incorrectly. However, the names give correct structural formulas. Draw structural formulas for the compounds, and then write the correct IUPAC name for each alcohol.
- a. 2-Ethyl-1-propanol
- b. 2,4-Butanediol
- c. 2-Methyl-3-butanol
- d. 1,4-Cyclopentanediol
(a)
Interpretation:
Structural formula has to be written for the given alcohols and correct IUPAC name has to be assigned.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
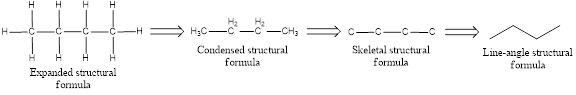
IUPAC rules for naming alcohols that contain single hydroxyl group:
- Longest carbon chain has to be identified that contains hydroxyl group also. The chain name is obtained by replacing the letter “-e” in alkane with “-ol”.
- The numbering has to be given so that the hydroxyl group gets the least numbering.
- Name and location of any other substituent present in the chain has to be identified.
- If in a ring the hydroxyl group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
- Hydroxyl group as a substituent in a molecule is named as hydroxy group rather than hydroxyl group.
IUPAC rules for naming alcohols that contain more than one hydroxyl group:
- The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of hydroxyl groups that is present.
Answer to Problem 14.19EP
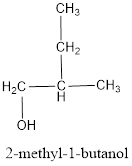
The structural formula is,
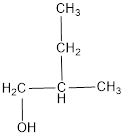
The correct IUPAC name of the given alcohol is 2-methyl-1-butanol.
Explanation of Solution
Given name of alcohol is 2-ethyl-1-propanol.
From the name it is identified that the parent alkane is propane with a hydroxyl group on first carbon atom and an ethyl group on second carbon atom.

The structural formula for the given alcohol is drawn as shown above.
IUPAC name can be identified by finding the longest continuous carbon chain with the hydroxyl group. In this case it is found to be a four carbon chain and hence the parent is butane. As the structure has a hydroxyl group in it, the suffix “-ol” has to be added instead of “-e” in the parent alkane. The numbering has to be given in a way that the hydroxyl group gets the least numbering. Looking for the substituent, a methyl group is present on the second carbon atom. This gives the IUPAC name of the alcohol as 2-methyl-1-butanol as hydroxyl is in the first carbon atom.
The structural formula for the given alcohol is drawn and correct IUPAC name is assigned.
(b)
Interpretation:
Structural formula has to be written for the given alcohols and correct IUPAC name has to be assigned.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
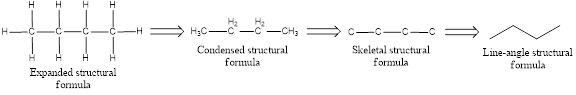
IUPAC rules for naming alcohols that contain single hydroxyl group:
- Longest carbon chain has to be identified that contains hydroxyl group also. The chain name is obtained by replacing the letter “-e” in alkane with “-ol”.
- The numbering has to be given so that the hydroxyl group gets the least numbering.
- Name and location of any other substituent present in the chain has to be identified.
- If in a ring the hydroxyl group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
- Hydroxyl group as a substituent in a molecule is named as hydroxy group rather than hydroxyl group.
IUPAC rules for naming alcohols that contain more than one hydroxyl group:
- The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of hydroxyl groups that is present.
Answer to Problem 14.19EP
The structural formula is,
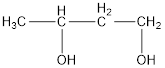
The correct IUPAC name of the given alcohol is 1,3-butanediol.
Explanation of Solution
Given name of alcohol is 2,4-butanediol.
From the name it is identified that there are two hydroxyl groups present each on second and fourth carbon atom of the parent alkane, butane. The structure can be drawn as,

The structural formula for the given alcohol is drawn as shown above.
IUPAC name can be identified by finding the longest continuous carbon chain with the hydroxyl group. In this case it is found to be a four carbon chain and hence the parent is butane. As the structure has two hydroxyl groups in it, the suffix “-diol” has to be added to the name of parent alkane. The numbering has to be given in a way that the hydroxyl group gets the least numbering. This gives the IUPAC name of the alcohol as 1,3-butanediol.
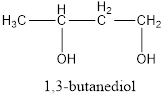
The structural formula for the given alcohol is drawn and correct IUPAC name is assigned.
(c)
Interpretation:
Structural formula has to be written for the given alcohols and correct IUPAC name has to be assigned.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
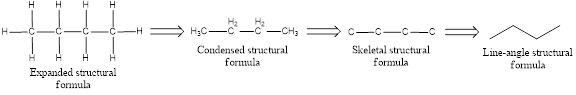
IUPAC rules for naming alcohols that contain single hydroxyl group:
- Longest carbon chain has to be identified that contains hydroxyl group also. The chain name is obtained by replacing the letter “-e” in alkane with “-ol”.
- The numbering has to be given so that the hydroxyl group gets the least numbering.
- Name and location of any other substituent present in the chain has to be identified.
- If in a ring the hydroxyl group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
- Hydroxyl group as a substituent in a molecule is named as hydroxy group rather than hydroxyl group.
IUPAC rules for naming alcohols that contain more than one hydroxyl group:
- The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of hydroxyl groups that is present.
Answer to Problem 14.19EP
The structural formula is,
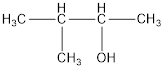
The correct IUPAC name of the given alcohol is 3-methyl-2-butanol.
Explanation of Solution
Given name of alcohol is 2-methyl-3-butanol.
From the name it is identified that the parent alkane is butane with a methyl group substituted in second carbon atom and a hydroxyl functional group on third carbon atom. The structure can be drawn as,

The structural formula for the given alcohol is drawn as shown above.
IUPAC name can be identified by finding the longest continuous carbon chain with the hydroxyl group. In this case it is found to be a four carbon chain and hence the parent is butane. As the structure has a hydroxyl group in it, the suffix “-ol” has to be added instead of “-e” in the parent alkane. The numbering has to be given in a way that the hydroxyl group gets the least numbering. Looking for the substituents, a methyl group is present in the third carbon atom. This gives the IUPAC name of the alcohol as 3-methyl-2-butanol as hydroxyl is in the second carbon atom.
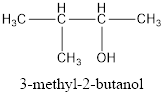
The structural formula for the given alcohol is drawn and correct IUPAC name is assigned.
(d)
Interpretation:
Structural formula has to be written for the given alcohols and correct IUPAC name has to be assigned.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
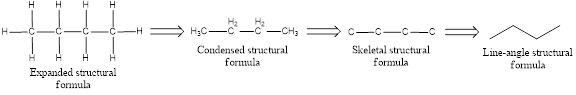
IUPAC rules for naming alcohols that contain single hydroxyl group:
- Longest carbon chain has to be identified that contains hydroxyl group also. The chain name is obtained by replacing the letter “-e” in alkane with “-ol”.
- The numbering has to be given so that the hydroxyl group gets the least numbering.
- Name and location of any other substituent present in the chain has to be identified.
- If in a ring the hydroxyl group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
- Hydroxyl group as a substituent in a molecule is named as hydroxy group rather than hydroxyl group.
IUPAC rules for naming alcohols that contain more than one hydroxyl group:
- The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of hydroxyl groups that is present.
Answer to Problem 14.19EP
The structural formula is,
The correct IUPAC name of the given alcohol is 1,3-cyclopentanediol.
Explanation of Solution
Given name of alcohol is 1,4-cyclopentanediol.
From the name it is identified that the parent cycloalkane is cyclopentane with two hydroxyl groups at first and fourth carbon atom. The structure can be drawn as,

The structural formula for the given alcohol is drawn as shown above.
IUPAC name can be identified by finding the longest continuous carbon chain with the hydroxyl group. In this case it is found to be a five carbon cyclic chain and hence the parent is cyclopentane. As the structure has two hydroxyl groups in it, the suffix “-diol” has to be added. The numbering has to be given in a way that the hydroxyl group gets the least numbering. This gives the IUPAC name of the alcohol as 1,3-cyclopentanediol as hydroxyl groups are in the first and third carbon atom.
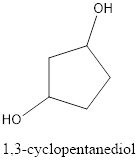
The structural formula for the given alcohol is drawn and correct IUPAC name is assigned.
Want to see more full solutions like this?
Chapter 14 Solutions
GENERAL,ORGANIC,+BIO.CHEM.-MINDTAP
- a. H3C. N H3C CH3 HCNarrow_forwardол 2. восцапан (46:00) Curtius rearrangment 1. NaN3, heat -OHarrow_forwardQuestion 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forward
- At an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forwardExplain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.arrow_forward
- Explain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





