
(a)
Interpretation:
From the given depiction, which shows the shapes of the reactants and product has to be given.
Concept Introduction:
Valence shell electron pair repulsion (VSEPR) theory:
The geometry of molecule is predicted by valence shell electron pair repulsion (VSEPR) theory, this theory state that the repulsion force between lone pair and bond pair electrons of central atom is given by,
Lone pair-lone pair > lone pair - bond pair > bond pair - bond pair
From the order of repulsion forces and number of lone pair electrons, bond pair electrons that are present in the central atom, the geometry of molecule is assigned in the below manner,
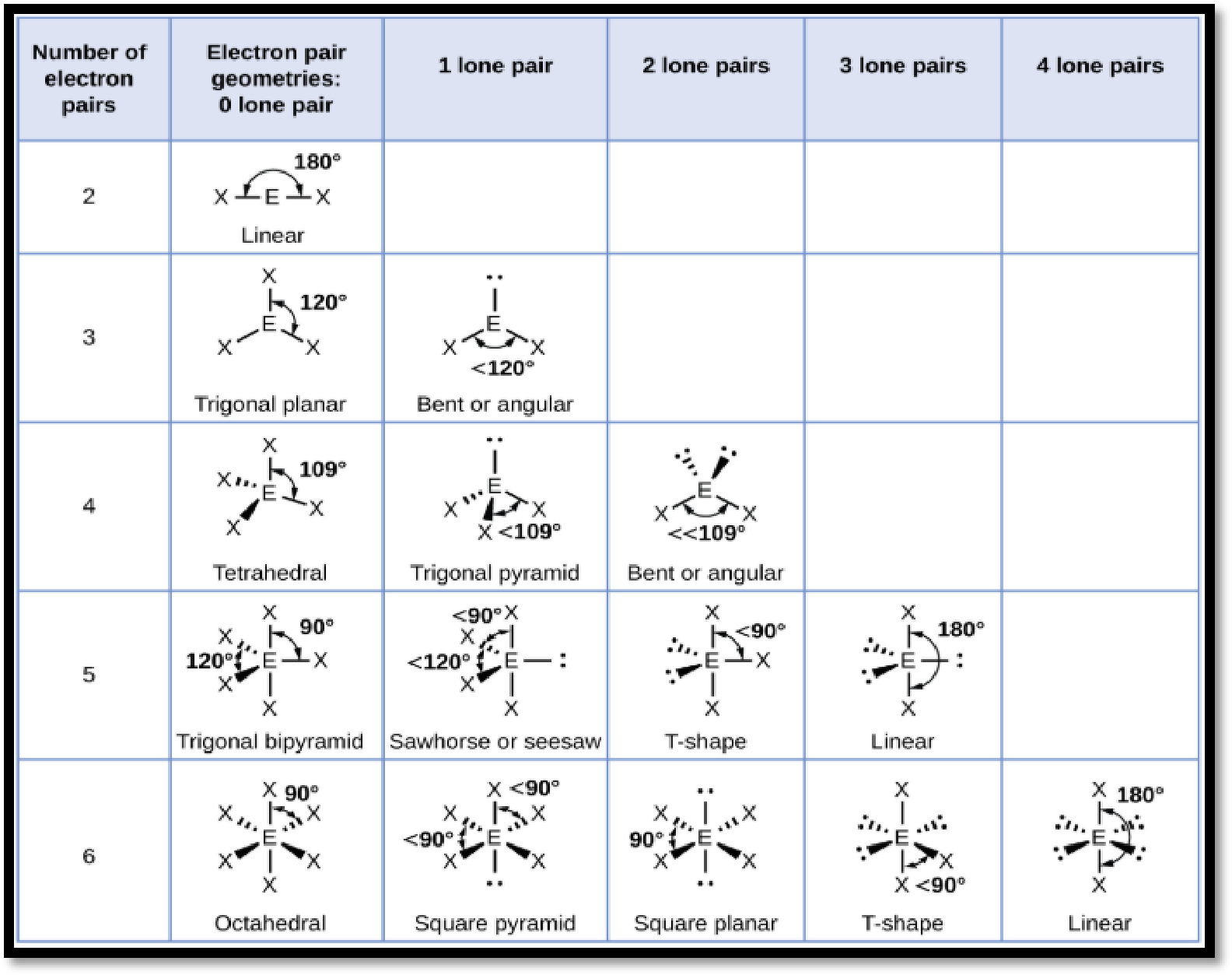
Figure 1
(c)
Interpretation:
The changes in hybridization of xenon in given reaction has to be given.
Concept Introduction:
Hybridization:
The formation of new orbitals from the mixing of atomic orbitals is known as hybridization.
In hybridization, energy and count of newly formed orbital is same as the atomic orbitals.
Overlapping of atomic orbitals are formed new hybridized molecular orbitals.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
CHEMISTRY:MOLEC NAT PRINT COMPANION
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





