
Concept explainers
(a)
Interpretation: The stereocenters present in the molecule needs to be labelled as R or S.
Concept Introduction:
The Cahn-Ingold-Prelog system is a set of rules that allows us to unambiguously define the stereochemical configuration of any stereocenter, using the designations 'R ' (from the Latin rectus, meaning right-handed) or ' S ' (from the Latin sinister, meaning left-handed).
In the first step, priority is given to 4 different groups attached to the stereocenter. The direction of 4th priority group should be away from the observer. In the second step, direction from 1 to 3 priority group is determined. If the direction is clockwise, the configuration will be R and if it is anticlockwise, the configuration will be S.
(a)
Explanation of Solution
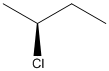
The given structure can be represented as follows:
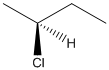
Here, stereocenter and priority in groups is labelled as follows:
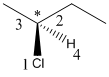
Here, the lowest priority group is H atom and it is bonded to C atom via dashed bond thus, it is away from us.
Now, determine the direction from 1 to 3 priority group. If the direction is clockwise, the configuration will be R and if it is anticlockwise, the configuration will be S.
Here, the direction is represented as follows:
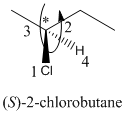
Since, it is anticlockwise, the configuration will be S.
(b)
Interpretation: The stereocenters present in the molecule needs to be labelled as R or S.
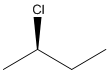
Concept Introduction:
The Cahn-Ingold-Prelog system is a set of rules that allows us to unambiguously define the stereochemical configuration of any stereocenter, using the designations 'R ' (from the Latin rectus, meaning right-handed) or ' S ' (from the Latin sinister, meaning left-handed).
In the first step, priority is given to 4 different groups attached to the stereocenter. The direction of 4th priority group should be away from the observer. In the second step, direction from 1 to 3 priority group is determined. If the direction is clockwise, the configuration will be R and if it is anticlockwise, the configuration will be S.
(b)
Explanation of Solution
The given structure can be represented as follows:
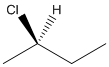
Here, stereocenter and priority in groups is labelled as follows:
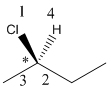
Here, the lowest priority group is H atom and it is bonded to C atom via dashed bond thus, it is away from us.
Now, determine the direction from 1 to 3 priority group. If the direction is clockwise, the configuration will be R and if it is anticlockwise, the configuration will be S.
Here, the direction is represented as follows:
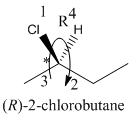
Since, it is clockwise, the configuration will be R.
(c)
Interpretation: The stereocenters present in the molecule needs to be labelled as R or S.
Concept Introduction:
The Cahn-Ingold-Prelog system is a set of rules that allows us to unambiguously define the stereochemical configuration of any stereocenter, using the designations 'R ' (from the Latin rectus, meaning right-handed) or ' S ' (from the Latin sinister, meaning left-handed).
In the first step, priority is given to 4 different groups attached to the stereocenter. The direction of 4th priority group should be away from the observer. In the second step, direction from 1 to 3 priority group is determined. If the direction is clockwise, the configuration will be R and if it is anticlockwise, the configuration will be S.
(c)
Explanation of Solution
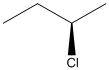
The given structure can be represented as follows:
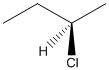
Here, stereocenter and priority in groups is labelled as follows:
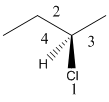
Here, the lowest priority group is H atom and it is bonded to C atom via dashed bond thus, it is away from us.
Now, determine the direction from 1 to 3 priority group. If the direction is clockwise, the configuration will be R and if it is anticlockwise, the configuration will be S.
Here, the direction is represented as follows:
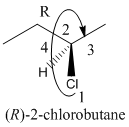
Since, it is clockwise, the configuration will be R.
(d)
Interpretation: The stereocenters present in the molecule needs to be labelled as R or S.
Concept Introduction:
The Cahn-Ingold-Prelog system is a set of rules that allows us to unambiguously define the stereochemical configuration of any stereocenter, using the designations 'R ' (from the Latin rectus, meaning right-handed) or ' S ' (from the Latin sinister, meaning left-handed).
In the first step, priority is given to 4 different groups attached to the stereocenter. The direction of 4th priority group should be away from the observer. In the second step, direction from 1 to 3 priority group is determined. If the direction is clockwise, the configuration will be R and if it is anticlockwise, the configuration will be S.
(d)
Explanation of Solution
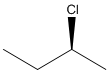
The given structure can be represented as follows:
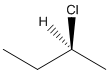
Here, stereocenter and priority in groups is labelled as follows:
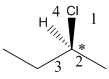
Here, the lowest priority group is H atom and it is bonded to C atom via dashed bond thus, it is away from us.
Now, determine the direction from 1 to 3 priority group. If the direction is clockwise, the configuration will be R and if it is anticlockwise, the configuration will be S.
Here, the direction is represented as follows:
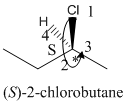
Since, it is anticlockwise, the configuration will be S.
(e)
Interpretation: The stereocenters present in the molecule needs to be labelled as R or S.
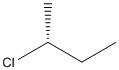
Concept Introduction:
The Cahn-Ingold-Prelog system is a set of rules that allows us to unambiguously define the stereochemical configuration of any stereocenter, using the designations 'R ' (from the Latin rectus, meaning right-handed) or ' S ' (from the Latin sinister, meaning left-handed).
In the first step, priority is given to 4 different groups attached to the stereocenter. The direction of 4th priority group should be away from the observer. In the second step, direction from 1 to 3 priority group is determined. If the direction is clockwise, the configuration will be R and if it is anticlockwise, the configuration will be S.
(e)
Explanation of Solution
The given structure can be represented as follows:
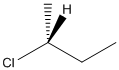
Here, stereocenter and priority in groups is labelled as follows:
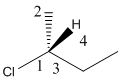
Here, the lowest priority group is H atom and it is bonded to C atom via wedge bond thus, it is towards us.
Now, determine the direction from 1 to 3 priority group. If the direction is clockwise, the configuration will be R and if it is anticlockwise, the configuration will be S.
Here, the direction is represented as follows:
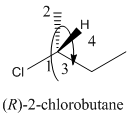
Here, the direction is clockwise thus, the configuration is R.
(f)
Interpretation: The stereocenters present in the molecule needs to be labelled as R or S.
Concept Introduction:
The Cahn-Ingold-Prelog system is a set of rules that allows us to unambiguously define the stereochemical configuration of any stereocenter, using the designations 'R ' (from the Latin rectus, meaning right-handed) or ' S ' (from the Latin sinister, meaning left-handed).
In the first step, priority is given to 4 different groups attached to the stereocenter. The direction of 4th priority group should be away from the observer. In the second step, direction from 1 to 3 priority group is determined. If the direction is clockwise, the configuration will be R and if it is anticlockwise, the configuration will be S.
(f)
Explanation of Solution
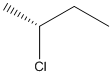
The given structure can be represented as follows:
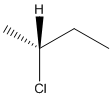
Here, stereocenter and priority in groups is labelled as follows:
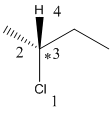
Here, the lowest priority group is H atom and it is bonded to C atom via wedge bond thus, it is towards us.
Now, determine the direction from 1 to 3 priority group. If the direction is clockwise, the configuration will be R and if it is anticlockwise, the configuration will be S.
Here, the direction is represented as follows:
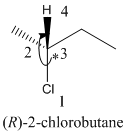
Here, the direction is clockwise thus, the configuration is R.
Want to see more full solutions like this?
Chapter 14 Solutions
Introduction to General, Organic and Biochemistry
- The two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forwardасть Identify all the bonds that gauche interact with C-OMe in the most stable conformation of the above compound.arrow_forwardPredict the reactants used in the formation of the following compounds using Acid-Catalyzed dehydration reactionarrow_forward
- Can I please get help with this?arrow_forward.. Give the major organic product(s) for each of the following reactions or sequences of reactions. Show ll relevant stereochemistry [3 ONLY]. A H Br 1. NaCN 2 NaOH, H₂O, heat 3. H3O+ B. CH₂COOH 19000 1. LiAlH4 THF, heat 2 H₂O* C. CH Br 1. NaCN, acetone 2 H3O+, heat D. Br 1. Mg. ether 3. H₂O+ 2 CO₂ E. CN 1. (CH) CHMgBr, ether 2 H₂O+arrow_forwardAssign this COSY spectrumarrow_forward
- Can I please get help with this?arrow_forward1. Draw structures corresponding to each of the following names [3 ONLY]: A. 2,2,2-trichloroethanal (chloral). B. trans-3-isopropylcyclohexanecarbaldehyde C. What is the correct structure for 2-hydroxyacetophenone? Circle the letter of your response. a C 0 OH OH OH HO b. H3C CH 0 H d OH D. Provide IUPAC names for each structure below. 0 H C-H 0 0 CH3 H NO₂ E. The substance formed on addition of water to an aldehyde or ketone is called a hydrate or a/an: a. vicinal diol b. geminal diol C. acetal d. ketalarrow_forwardAssign this spectrumarrow_forward
- Redraw the tripeptide with or without its acidic hydrogensto demonstrate where the total charge of -2 comes from: *see imagearrow_forward2. Consider the data below to answer the following questions. Cyanohydrins are important intermediates in the synthesis of α-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. Unfortunately, when a cyanohydrin is treated with aqueous base the original carbonyl compound is isolated. OH CH-COOH 0 HO CN C H30* C. H H HC N NaOH H₂O C=O 0 cyanohydrin H + NaCN + H₂Oarrow_forwardAssign all integrated peaksarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
