
(a)
Interpretation: A starting material and reagents used to form following epoxide needs to be identified.
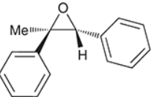
Concept introduction:
Preparation of epoxide-
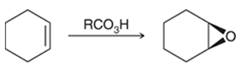
Peroxy acids generally used in this process are MCPBA and peroxyacetic acid. Formation of epoxide via peroxy acid us a stereospecific process thus, cis substituents in alkene (starting material) remain at cis to each other in the epoxide (product). Similarly, trans substituents in alkene remain at trans to each other.
(b)
Interpretation: A starting material and reagents used to form following epoxide needs to be identified.
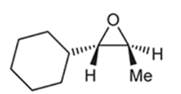
Concept introduction:
Preparation of epoxide- Epoxides can be prepared using peroxy acids. Alkenes are starting material in the preparation of epoxides with peroxy acids. Here, general reaction is represented as follows:
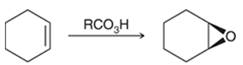
Peroxy acids generally used in this process are MCPBA and peroxyacetic acid. Formation of epoxide via peroxy acid us a stereospecific process thus, cis substituents in alkene (starting material) remain at cis to each other in the epoxide (product). Similarly, trans substituents in alkene remain at trans to each other.
(c)
Interpretation: A starting material and reagents used to form following epoxide needs to be identified.
Concept introduction:
Preparation of epoxide- Epoxides can be prepared using peroxy acids. Alkenes are starting material in the preparation of epoxides with peroxy acids. Here, general reaction is represented as follows:
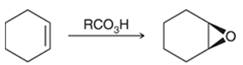
Peroxy acids generally used in this process are MCPBA and peroxyacetic acid. Formation of epoxide via peroxy acid us a stereospecific process thus, cis substituents in alkene (starting material) remain at cis to each other in the epoxide (product). Similarly, trans substituents in alkene remain at trans to each other.
(d)
Interpretation: A starting material and reagents used to form following epoxide needs to be identified.
Concept introduction:
Preparation of epoxide- Epoxides can be prepared using peroxy acids. Alkenes are starting material in the preparation of epoxides with peroxy acids. Here, general reaction is represented as follows:
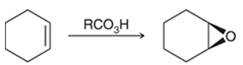
Peroxy acids generally used in this process are MCPBA and peroxyacetic acid. Formation of epoxide via peroxy acid us a stereospecific process thus, cis substituents in alkene (starting material) remain at cis to each other in the epoxide (product). Similarly, trans substituents in alkene remain at trans to each other.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
ORGANIC CHEMISTRY-PRINT MULTI TERM
- Calculate the ionization energy of He+ and Li²+ ions in their ground states. Thannnxxxxx sirrr Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdvaarrow_forwardPlleeaasseee solllveeee question 3 andd thankss sirr, don't solve it by AI plleeaasseee don't use AIarrow_forwardCalculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forward
- 4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardIII O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward
- 3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forwardWhat is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

