
Thermodynamics: An Engineering Approach
9th Edition
ISBN: 9781259822674
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 13.3, Problem 33P
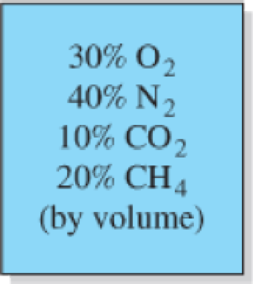
The volumetric analysis of a mixture of gases is 30 percent oxygen, 40 percent nitrogen, 10 percent carbon dioxide, and 20 percent methane. Calculate the apparent specific heats and molecular weight of this mixture of gases.
FIGURE P13–33
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Calculate for the vertical cross section moment of inertia for both Orientations 1 and 2 of a 1 x 1.5 in. horizontal hollow rectangular beam with wall thickness of t = 0.0625 in.
Use the equation: I = ((bh^3)/12) - (((b-2t)(h-2t)^3)/12)
Please answer 'yes' or 'no' and 'is' or 'is not' for the following:
Consider a large 23-cm-thick stainless steel plate (k = 15.1 W/m-K) in which heat is generated uniformly at a rate of 5 x 105
W/m³. Both sides of the plate are exposed to an environment at 30°C with a heat transfer coefficient of 60 W/m²K.
The highest temperature will occur at surfaces of plate while the lowest temperature will occur at the midplane.
Yes or No
Yes
No
Chapter 13 Solutions
Thermodynamics: An Engineering Approach
Ch. 13.3 - What are mass and mole fractions?Ch. 13.3 - Consider a mixture of several gases of identical...Ch. 13.3 - The sum of the mole fractions for an ideal-gas...Ch. 13.3 - Somebody claims that the mass and mole fractions...Ch. 13.3 - Consider a mixture of two gases. Can the apparent...Ch. 13.3 - What is the apparent molar mass for a gas mixture?...Ch. 13.3 - Prob. 7PCh. 13.3 - The composition of moist air is given on a molar...Ch. 13.3 - Prob. 9PCh. 13.3 - Prob. 10P
Ch. 13.3 - A gas mixture consists of 20 percent O2, 30...Ch. 13.3 - Prob. 12PCh. 13.3 - Prob. 13PCh. 13.3 - Consider a mixture of two gases A and B. Show that...Ch. 13.3 - Is a mixture of ideal gases also an ideal gas?...Ch. 13.3 - Express Daltons law of additive pressures. Does...Ch. 13.3 - Express Amagats law of additive volumes. Does this...Ch. 13.3 - Prob. 18PCh. 13.3 - How is the P-v-T behavior of a component in an...Ch. 13.3 - Prob. 20PCh. 13.3 - Prob. 21PCh. 13.3 - Prob. 22PCh. 13.3 - Consider a rigid tank that contains a mixture of...Ch. 13.3 - Prob. 24PCh. 13.3 - Is this statement correct? The temperature of an...Ch. 13.3 - Is this statement correct? The volume of an...Ch. 13.3 - Is this statement correct? The pressure of an...Ch. 13.3 - A gas mixture at 300 K and 200 kPa consists of 1...Ch. 13.3 - Prob. 29PCh. 13.3 - Separation units often use membranes, absorbers,...Ch. 13.3 - Prob. 31PCh. 13.3 - The mass fractions of a mixture of gases are 15...Ch. 13.3 - The volumetric analysis of a mixture of gases is...Ch. 13.3 - An engineer has proposed mixing extra oxygen with...Ch. 13.3 - A rigid tank contains 0.5 kmol of Ar and 2 kmol of...Ch. 13.3 - A mixture of gases consists of 0.9 kg of oxygen,...Ch. 13.3 - Prob. 37PCh. 13.3 - One pound-mass of a gas whose density is 0.001...Ch. 13.3 - A 30 percent (by mass) ethane and 70 percent...Ch. 13.3 - Prob. 40PCh. 13.3 - Prob. 41PCh. 13.3 - A rigid tank that contains 2 kg of N2 at 25C and...Ch. 13.3 - Prob. 43PCh. 13.3 - Prob. 44PCh. 13.3 - Prob. 45PCh. 13.3 - Is the total internal energy of an ideal-gas...Ch. 13.3 - Prob. 47PCh. 13.3 - Prob. 48PCh. 13.3 - Prob. 49PCh. 13.3 - Prob. 50PCh. 13.3 - The volumetric analysis of a mixture of gases is...Ch. 13.3 - A mixture of nitrogen and carbon dioxide has a...Ch. 13.3 - The mass fractions of a mixture of gases are 15...Ch. 13.3 - A mixture of gases consists of 0.1 kg of oxygen, 1...Ch. 13.3 - An insulated tank that contains 1 kg of O2at 15C...Ch. 13.3 - An insulated rigid tank is divided into two...Ch. 13.3 - Prob. 59PCh. 13.3 - A mixture of 65 percent N2 and 35 percent CO2...Ch. 13.3 - Prob. 62PCh. 13.3 - Prob. 63PCh. 13.3 - Prob. 66PCh. 13.3 - Prob. 67PCh. 13.3 - Prob. 68PCh. 13.3 - Prob. 69PCh. 13.3 - The gas passing through the turbine of a simple...Ch. 13.3 - Prob. 71PCh. 13.3 - A pistoncylinder device contains 6 kg of H2 and 21...Ch. 13.3 - Prob. 73PCh. 13.3 - Prob. 74PCh. 13.3 - Prob. 75PCh. 13.3 - Prob. 76PCh. 13.3 - Prob. 77PCh. 13.3 - Prob. 78PCh. 13.3 - Prob. 79PCh. 13.3 - Prob. 81PCh. 13.3 - Fresh water is obtained from seawater at a rate of...Ch. 13.3 - Is it possible for an adiabatic liquid-vapor...Ch. 13.3 - Prob. 84PCh. 13.3 - Prob. 85RPCh. 13.3 - The products of combustion of a hydrocarbon fuel...Ch. 13.3 - A mixture of gases is assembled by first filling...Ch. 13.3 - Prob. 90RPCh. 13.3 - Prob. 91RPCh. 13.3 - Prob. 92RPCh. 13.3 - A rigid tank contains a mixture of 4 kg of He and...Ch. 13.3 - A spring-loaded pistoncylinder device contains a...Ch. 13.3 - Prob. 95RPCh. 13.3 - Reconsider Prob. 1395. Calculate the total work...Ch. 13.3 - Prob. 97RPCh. 13.3 - Prob. 100RPCh. 13.3 - Prob. 101RPCh. 13.3 - Prob. 102FEPCh. 13.3 - An ideal-gas mixture whose apparent molar mass is...Ch. 13.3 - An ideal-gas mixture consists of 2 kmol of N2and 4...Ch. 13.3 - Prob. 105FEPCh. 13.3 - Prob. 106FEPCh. 13.3 - An ideal-gas mixture consists of 3 kg of Ar and 6...Ch. 13.3 - Prob. 108FEPCh. 13.3 - Prob. 109FEPCh. 13.3 - Prob. 110FEPCh. 13.3 - Prob. 111FEP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- My answers are incorrectarrow_forwardPicturearrow_forwardWhat is the weight of a 5-kg substance in N, kN, kg·m/s², kgf, Ibm-ft/s², and lbf? The weight of a 5-kg substance in N is 49.05 N. The weight of a 5-kg substance in kN is KN. The weight of a 5-kg substance in kg·m/s² is 49.05 kg-m/s². The weight of a 5-kg substance in kgf is 5.0 kgf. The weight of a 5-kg substance in Ibm-ft/s² is 11.02 lbm-ft/s². The weight of a 5-kg substance in lbf is 11.023 lbf.arrow_forward
- Mych CD 36280 kg. 0.36 givens Tesla truck frailer 2017 Model Vven 96154kph ronge 804,5km Cr Powertrain Across PHVAC rwheel 0.006 0.88 9M² 2 2kW 0.55M ng Zg Prated Trated Pair 20 0.95 1080 kW 1760 Nm 1,2 determine the battery energy required to meet the range when fully loaded determine the approximate time for the fully-loaded truck-trailor to accelerate from 0 to 60 mph while Ignoring vehicle load forcesarrow_forward12-217. The block B is sus- pended from a cable that is at- tached to the block at E, wraps around three pulleys, and is tied to the back of a truck. If the truck starts from rest when ID is zero, and moves forward with a constant acceleration of ap = 0.5 m/s², determine the speed of the block at D the instant x = 2 m. Neglect the size of the pulleys in the calcu- lation. When xƊ = 0, yc = 5 m, so that points C and D are at the Prob. 12-217 5 m yc =2M Xparrow_forwardsolve both and show matlab code auto controlsarrow_forward
- 12-82. The roller coaster car trav- els down the helical path at con- stant speed such that the paramet- ric equations that define its posi- tion are x = c sin kt, y = c cos kt, z = h - bt, where c, h, and b are constants. Determine the mag- nitudes of its velocity and accelera- tion. Prob. 12-82 Narrow_forwardGiven: = refueling Powertran SOURCE EMISSIONS vehide eff eff gasoline 266g co₂/kwh- HEV 0.90 0.285 FLgrid 411ilg Co₂/kWh 41111gCo₂/kWh EV 0.85 0.80 Production 11x10% og CO₂ 13.7 x 10°g CO₂ A) Calculate the breakeven pont (in km driven) for a EV against on HEV in Florida of 0.1kWh/kM Use a drive cycle conversion 5) How efficient would the powertrain of the HEV in this example have to be to break even with an EV in Florida after 150,000 Miles of service (240,000) km Is it plausible to achieve the answer from pert b Consideans the HaXINERY theoretical efficiency of the Carnot cycle is 5020 and there are additional losses of the transMISSION :- 90% efficiency ? c A what do you conclude is the leading factor in why EVs are less emissive than ICE,arrow_forwardsolve autocontrolsarrow_forward
- Problem 3.21P: Air at 100F(38C) db,65F(18C) wb, and sea-level pressure is humidified adiabatically with steam. The steam supplied contains 20 percent moisture(quality of 0.80) at 14.7psia(101.3kpa). The air is humidified to 60 percent relative humidity. Find the dry bulb temperature of the humidified air using (a)chart 1a or 1b and (b) the program PSYCH.arrow_forwardPUNTO 4. calculate their DoF using Gruebler's formula. PUNTO 5. Groundarrow_forwardPUNTO 2. PUNTO 3. calculate their DoF using Gruebler's formula. III IAarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Thermodynamics - Chapter 3 - Pure substances; Author: Engineering Deciphered;https://www.youtube.com/watch?v=bTMQtj13yu8;License: Standard YouTube License, CC-BY