
LL ORG CHEM
6th Edition
ISBN: 9781264840083
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 61P
Devise a synthesis of each compound using
a.
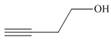 c.
c.  d.
d. 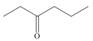
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Synthesize each compound from cyclohexanol, ethanol, and any other needed reagents.
Show how to bring about each conversion in good yield.
a.
b.
C6H5
Cl
OH
COOH
C6H5 COOH
cis-Cyclohexane-1,2-diol can be synthesized from cyclohexene by using which reagent?
a.O3
b.OsO4
c.H2SO4
d.mCPBA
Chapter 13 Solutions
LL ORG CHEM
Ch. 13.1 - Prob. 1PCh. 13.1 - Prob. 2PCh. 13.2 - Prob. 3PCh. 13.3 - Prob. 4PCh. 13.3 - Prob. 5PCh. 13.4 - Prob. 7PCh. 13.5 - Problem 15.8 Which bond in the each compound is...Ch. 13.6 - Prob. 9PCh. 13.6 - Prob. 10PCh. 13.7 - Prob. 11P
Ch. 13.7 - Prob. 12PCh. 13.8 - Prob. 13PCh. 13.8 - Prob. 14PCh. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 34PCh. 13 - 15.37 What alkane is needed to make each alkyl...Ch. 13 - 15.38 Which alkyl halides can be prepared in good...Ch. 13 - Prob. 37PCh. 13 - 15.40 Explain why radical bromination of p-xylene...Ch. 13 - a. What product(s) (excluding stereoisomers) are...Ch. 13 - Prob. 40PCh. 13 - 15.43 Draw the products formed when each alkene is...Ch. 13 - 15.44 Draw all constitutional isomers formed when...Ch. 13 - 15.45 Draw the organic products formed in each...Ch. 13 - Prob. 45PCh. 13 - 15.47 Treatment of a hydrocarbon A (molecular...Ch. 13 - 15.48 Draw the products formed in each reaction...Ch. 13 - 15.53 Consider the following bromination: .
a....Ch. 13 - 15.54 Draw a stepwise mechanism for the following...Ch. 13 - Prob. 57PCh. 13 - 15.57 Devise a synthesis of each compound from...Ch. 13 - Prob. 59PCh. 13 - Prob. 60PCh. 13 - 15.60 Devise a synthesis of each compound using ...Ch. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - 15.63 As described in Section 9.16, the...Ch. 13 - 15.64 Ethers are oxidized with to form...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the organic products formed in each reaction.arrow_forwardSynthesize each compound from toluene (C6H5CH3) and any other organic or inorganic reagents.arrow_forwardb. d. a. a 6. Draw the organic product formed for each reaction. COOH COOH A [1] LDA [2] CH3CH₂l [1] Br₂. CH3CO₂H [2] Li₂CO3. LiBr. DMF 12 (excess) TOH CN NaH Br₂ (excess) TOH + CHI3arrow_forward
- Complete the reactions.arrow_forwardSynthesize each compound from benzene and any other organic or inorganic reagents. NH2 NO2 Br. a. е. NH2 Br CH3 Br NH2 b. d. HOOC NO2 (РАВA) sunscreen componentarrow_forwardSynthesize each compound from cyclohexanone and organic halides having s4 C's. You may use any other inorganic reagents. OCH3 a. C. OH b. d.arrow_forward
- Instructions: Give the IUPAC name for each compound. A. CH₂ CH₂CH₂CH₂CCH₂COOH CH₂ B. CH.CHCHCH.COOH a CH₂CH₂ C. (CH,CH,), CHCH,CH COOH Instructions: Give the structure corresponding to each IUPAC name. a. 2-bromobutanoic acid b. 2,3-dimethylpentanoic acid c. 2-ethyl-5,5-dimethyloctanoic acid d. 3,4,5,6-tetraethyldecanoic acidarrow_forwardI need help with question 3arrow_forwardCompound that is most easily hydrolyzed by acid in water `NH A. В. F C. D.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY