
LL ORG CHEM
6th Edition
ISBN: 9781264840083
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 38P
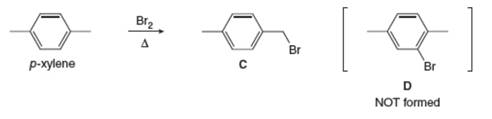
Explain why radical bromination of p-xylene forms
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Explain why radical bromination of p-xylene forms C rather than D ?
Draw the structure of the minor product formed in the reaction of 2-methylbut-1-ene with
concentrated sulfuric acid
Explain why thid is the major product?
Structure of minor product?
Explanation?
Why does 2,3-dimethyl-2-butene react faster than 2-methyl-2-butene?
Chapter 13 Solutions
LL ORG CHEM
Ch. 13.1 - Prob. 1PCh. 13.1 - Prob. 2PCh. 13.2 - Prob. 3PCh. 13.3 - Prob. 4PCh. 13.3 - Prob. 5PCh. 13.4 - Prob. 7PCh. 13.5 - Problem 15.8 Which bond in the each compound is...Ch. 13.6 - Prob. 9PCh. 13.6 - Prob. 10PCh. 13.7 - Prob. 11P
Ch. 13.7 - Prob. 12PCh. 13.8 - Prob. 13PCh. 13.8 - Prob. 14PCh. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 34PCh. 13 - 15.37 What alkane is needed to make each alkyl...Ch. 13 - 15.38 Which alkyl halides can be prepared in good...Ch. 13 - Prob. 37PCh. 13 - 15.40 Explain why radical bromination of p-xylene...Ch. 13 - a. What product(s) (excluding stereoisomers) are...Ch. 13 - Prob. 40PCh. 13 - 15.43 Draw the products formed when each alkene is...Ch. 13 - 15.44 Draw all constitutional isomers formed when...Ch. 13 - 15.45 Draw the organic products formed in each...Ch. 13 - Prob. 45PCh. 13 - 15.47 Treatment of a hydrocarbon A (molecular...Ch. 13 - 15.48 Draw the products formed in each reaction...Ch. 13 - 15.53 Consider the following bromination: .
a....Ch. 13 - 15.54 Draw a stepwise mechanism for the following...Ch. 13 - Prob. 57PCh. 13 - 15.57 Devise a synthesis of each compound from...Ch. 13 - Prob. 59PCh. 13 - Prob. 60PCh. 13 - 15.60 Devise a synthesis of each compound using ...Ch. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - 15.63 As described in Section 9.16, the...Ch. 13 - 15.64 Ethers are oxidized with to form...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For Sn1 and Sn2 reactions, explain why one type of carbon should react faster than others.arrow_forwardExplain Electrostatic potential maps of four halomethanes (CH3X)arrow_forwardRadical bromination of propene using NBS gives 3-bromo-1-propene. Draw the allylic radical intermediate formed during this reaction, showing both resonance structures.arrow_forward
- Define Nucleophilic Substitution of RCOZ (Z = Leaving Group) ?arrow_forwardDraw the major organic product formed by reaction of 2-hexyne with the following reagent: H₂O in H₂SO4 / HgSO4. • Consider E/Z stereochemistry of alkenes. • In cases where there is more than one answer, just draw one. • If no reaction occurs, draw the organic starting material.arrow_forward1. b) Show the MECHANISM for the POLAR addition of molecular chlorine to cyclopentane. c) The reaction of toluene and ethylbenzene was through the alkyl groups, -CH 3 and -CH 2 CH 3, respectively. Why didn’t xylene, which has TWO methyl groups, react with bromine in the time allotted?arrow_forward
- Draw the allylic bromination products formed when the alkene shown below reacts with NBS/uv light.arrow_forward8. Explain WHY the reaction of alkene with water/alcohol require acid, while the reaction of an alkene with Br2 does not.arrow_forwardExplain the relative reaction rates of addition to alkenes of HCl, HBr, and HI.arrow_forward
- Explain how Hammond's postulate accounts for the higher selectivity of bromination reactions as compared to chlorination reactions.arrow_forwardDraw the product formed when each triene undergoes electrocyclic reaction under [1] thermal conditions; [2] photochemical conditions. b.arrow_forwardtert-Butylbenzene can be prepared by alkylation of benzene using an alkene or an alcohol as the carbocation source. What alkene? What alcohol?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License