
Chemistry In Focus
6th Edition
ISBN: 9781305544727
Author: Tro
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 52E
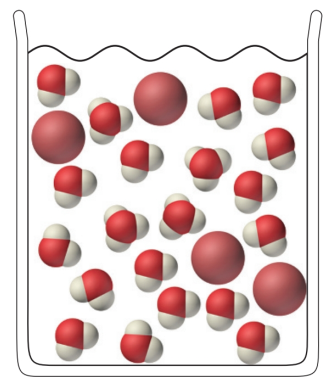
Determine from the following molecular view of a hydrobromic acid (HBr) solution whether HBr is a strong or a weak acid:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Question 5: Name the following compound in two ways
using side chain and using prefix amine (Common name
and IUPAC name both)
CH3NH2
CH3CH2NHCH3
CH₂CH₂N(CH3)2
Draw the structure of diethyl methyl amine
Question 6. Write the balanced combustion reaction
for:
a. Hexane
b. Propyne
c. 2-pentene
Question 7: Write the following electrophilic
substitution reactions of benzene:
Hint: Use notes if you get confused
a. Halogenation reaction:
b. Nitration reaction :
c. Sulphonation reaction:
d. Alkylation reaction:
e. Aceylation reaction:
Question 4. Name the following structures
○
CH3-C-N-H
H
CH3CH2-C-N-H
H
CH3CH2-C-N-CH3
H
A. Add Water to below compound which 2-methyl 2-butene (addition Reaction)
H₂C
CH₂
CH,
+ H₂O-> ?
Major product?
Minor product?
B. Add Bromine to the compound which 2-methyl 2-butene (addition Reaction)
CH₂
CH₂
+ Br₂→ ?
Major product and Minor product both are same in this?
C. Add Hydrogen Bromide to the compound which 2-methyl 2-butene (addition
Reaction)
H,C
CH₂
CH₂
+ HBr
Major product?
Minor product?
D. Add Hydrogen to the compound which 2-methyl 2-butene (addition Reaction)
CH₂
CH₂
+ H₂
Major product and Minor product both are same in this?
Chapter 13 Solutions
Chemistry In Focus
Ch. 13 - Which property is not generally associated with...Ch. 13 - Prob. 2SCCh. 13 - The ideal pH of a swimming pool is 7.2. You...Ch. 13 - Prob. 13.1YTCh. 13 - Identify the Brnsted-Lowry acid and base in the...Ch. 13 - Prob. 1ECh. 13 - What are the properties of acids?Ch. 13 - Prob. 3ECh. 13 - Prob. 4ECh. 13 - List five common laboratory acids and their uses.
Ch. 13 - Why are bases not commonly found in foods?Ch. 13 - List four common laboratory bases and their uses.Ch. 13 - What are the Arrhenius definitions of acids and...Ch. 13 - What are the Brnsted-Lowry definitions of acids...Ch. 13 - What is the difference between a strong acid and a...Ch. 13 - Prob. 11ECh. 13 - What pH range is considered acidic? Basic?...Ch. 13 - What acid is responsible for the sour taste of...Ch. 13 - What is pickling? What acid is responsible for the...Ch. 13 - Prob. 15ECh. 13 - Prob. 16ECh. 13 - List several common acids and where they might be...Ch. 13 - Prob. 18ECh. 13 - Prob. 19ECh. 13 - What causes acid indigestion? List some common...Ch. 13 - Prob. 21ECh. 13 - Explain how a leavening agent works.Ch. 13 - Prob. 23ECh. 13 - Prob. 24ECh. 13 - Prob. 25ECh. 13 - Prob. 26ECh. 13 - Prob. 27ECh. 13 - Prob. 28ECh. 13 - Write a chemical equation to show the...Ch. 13 - Write a chemical equation to show the...Ch. 13 - Identify the Brnsted-Lowry acid and base in each...Ch. 13 - Identify the Brnsted-Lowry acid and base in each...Ch. 13 - Write a chemical equation using Lewis structures...Ch. 13 - Write a chemical equation using Lewis structures...Ch. 13 - A chemist makes two solutions. One is a 0.01-MHCl...Ch. 13 - A chemist makes a 0.001-MNaOH solution and a...Ch. 13 - Give the pH that corresponds to each solution and...Ch. 13 - Give the pH that corresponds to each solution and...Ch. 13 - What is the [H3O+] in a solution with a pH of 4?Ch. 13 - What is the [H3O+] in a solution with a pH of 11?Ch. 13 - Write chemical reactions to show how each antacid...Ch. 13 - Write chemical reactions to show how each antacid...Ch. 13 - Suppose that the stomach contains...Ch. 13 - Suppose that 250.0 mL of a basic solution is 0.100...Ch. 13 - Prob. 45ECh. 13 - Write a chemical reaction to show how NO2 forms...Ch. 13 - Prob. 47ECh. 13 - Prob. 50ECh. 13 - Determine from the following molecular view of a...Ch. 13 - Determine from the following molecular view of a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 36) Complete the following multi-step reactions showing applications of enolate ions arising from ketones, esters, malonic ester, and keto ester, etc. (30 pts) (1) A NaOH, H₂O+ heat A NaOEt EtO OEt (11) EOH, H+ H. B LDA, H₂O+ -78°C B (i) NaOMe, Et-Br (ii) H₂O+, heat EtOOC (III) COOEt B A (i) NaOEt LiAlH 4-bromo-2-butene H₂O+ (ii) H3O+, heat Write the mechanism for Aldol Condensation (I A or B), and Claisen Condensation (II A).arrow_forward31) Complete two sets of reactions involving (R)-4-methyl-pent-2-ol producing racemic mixture of tertiary alcohols (D) and ketone derivative (C). Illustrate the mechanism of B and C or D. (25 pts) O OH 0 K2Cr2O7 Ph-CH2-Br, Mg, H2SO4 THF, H3O* (A) (D) Racemic mixture TsCl, Py (B) KCN, DMSO Ph-CH2-Br, Mg, THF, H3O+ (C) Mechanism for reactions B and C:arrow_forwardManoharan Mariappan, Ph.D., Dept. of Natur. Sci., NFC, Tallahassee, FL 33) Synthesize the aromatic compound containing para-substituted carbonyl compound starting from benzene. Illustrate the mechanism for reaction A. 1) NU (25 pts) A FeCl B (i) HNO3, H2SO4 (II) Sn, HCl(aq) NH₂ NO₂-D NH₂ (i) MeCO2Me, heat C (ii) K2Cr2O7/H2SO4 D (ii) SOCl2 (iii) 2 Et-NH2 Mechanism for reaction for the nitration of alkyl benzene (B-i): Characterize the product compound arising from the reaction D by IR and IH NMR spectral data: IR data (cm): 'H NMR data: Draw the structure and assign the chemical shift with the spin-splitting.arrow_forward
- Write structural formulas for the major products by doing addition reactions 1. You must add H2 as Pt is catalyst it does not take part in reactions only speed up the process H₂ CH2=CH-CH3 Pt 2. Add HCI break it into H and Cl CH3 HCI 3. Add Br2 only CC14 is catalyst CH3-CH=CH2 B12 CCl4 4. Add water to this and draw major product, H2SO4 is catalyst you have add water H20 in both the reaction below H₂SO4 CH3-CH=CH2 CH3 H2SO4/H₂O CH3-C=CH2 reflux ?arrow_forwardPlan the synthesis of the following compound using the starting material provided and any other reagents needed as long as carbon based reagents have 3 carbons or less. Either the retrosynthesis or the forward synthesis (mechanisms are not required but will be graded if provided) will be accepted if all necessary reagents and intermediates are shown (solvents and temperature requirements are not needed unless specifically involved in the reaction, i.e. DMSO in the Swern oxidation or heat in the KMnO4 oxidation). H Harrow_forwardHint These are benzene substitution reactions. ALCI3 and UV light are catalyst no part in reactions and triangle A means heating. A. Add ethyl for Et in benzene ring alkylation reaction EtCl = CH3CH2CL 1) EtC1 / AlCl3 / A ? B: Add Br to benzene ring ( substitution) 2) Br₂ / uv light ? C Add (CH3)2 CHCH2 in benzene ring ( substitution) (CH3)2CHCH,C1 / AICI, ?arrow_forward
- Draw the mechanism to make the alcohol 2-hexanol. Draw the Mechanism to make the alcohol 1-hexanol.arrow_forwardDraw the mechanism for the formation of diol by starting with 1-pentanal in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forwardIdentify each chiral carbon as either R or S. Identify the overall carbohydrates as L or Darrow_forward
- Ethers can be formed via acid-catalyzed acetal formation. Draw the mechanism for the molecule below and ethanol.arrow_forwardHOCH, H HO CH-OH OH H OH 11 CH₂OH F II OH H H 0 + H OHarrow_forwardDraw the mechanism for the formation of diol by starting with one pen and all in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY