
Connect with LearnSmart for Krauskopf: The Physical Universe, 16e
16th Edition
ISBN: 9781259663895
Author: KRAUSKOPF, Konrad B.
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 13, Problem 31E
To what class of organic compounds does the compound belong whose structure is shown below?
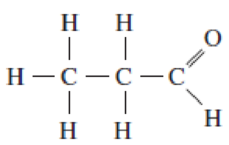
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Hi,
I have canceled, why did you charge me again?
No chatgpt pls will upvote
No chatgpt pls will upvote
Chapter 13 Solutions
Connect with LearnSmart for Krauskopf: The Physical Universe, 16e
Ch. 13 - Prob. 1MCCh. 13 - Prob. 2MCCh. 13 - Prob. 3MCCh. 13 - Prob. 4MCCh. 13 - As a class, the alkanes are a. highly reactive b....Ch. 13 - Prob. 6MCCh. 13 - Gasoline is a mixture of a. alkanes b. isomers of...Ch. 13 - Prob. 8MCCh. 13 - Unsaturated hydrocarbon molecules are...Ch. 13 - Prob. 10MC
Ch. 13 - Prob. 11MCCh. 13 - Prob. 12MCCh. 13 - Prob. 13MCCh. 13 - Prob. 14MCCh. 13 - Prob. 15MCCh. 13 - Prob. 16MCCh. 13 - Prob. 17MCCh. 13 - Prob. 18MCCh. 13 - Prob. 19MCCh. 13 - Prob. 20MCCh. 13 - Prob. 21MCCh. 13 - Prob. 22MCCh. 13 - Living cells consist mainly of a. carbohydrates b....Ch. 13 - Prob. 24MCCh. 13 - Prob. 25MCCh. 13 - Photosynthesis produces a. carbohydrates b....Ch. 13 - Prob. 27MCCh. 13 - Prob. 28MCCh. 13 - Prob. 29MCCh. 13 - Prob. 30MCCh. 13 - Prob. 31MCCh. 13 - Lipids are synthesized in plants and animals from...Ch. 13 - Prob. 33MCCh. 13 - Prob. 34MCCh. 13 - Proteins consist of combinations of a. amino acids...Ch. 13 - The number of amino acids important to life is a....Ch. 13 - Prob. 37MCCh. 13 - Prob. 38MCCh. 13 - Prob. 39MCCh. 13 - Prob. 40MCCh. 13 - Each three-nucleotide group in a DNA molecule...Ch. 13 - DNA is involved in which one or more of the...Ch. 13 - Prob. 1ECh. 13 - Prob. 2ECh. 13 - What is the principal bonding mechanism in organic...Ch. 13 - How can the different alkanes in petroleum be...Ch. 13 - Prob. 5ECh. 13 - Why are structural formulas more important in...Ch. 13 - The isomers of a compound have the same chemical...Ch. 13 - Distinguish between unsaturated and saturated...Ch. 13 - How many electrons are shared in a double bond...Ch. 13 - What kind of carbon-carbon bonds are found in...Ch. 13 - How many covalent bonds are present between the...Ch. 13 - In general, how do the reactivities of hydrocarbon...Ch. 13 - Prob. 13ECh. 13 - The alkanes of Sec. 13.2 are saturated...Ch. 13 - The structural formula of propane is given in Sec....Ch. 13 - Why does this structural formula not represent an...Ch. 13 - Why does this structural formula not represent an...Ch. 13 - Prob. 18ECh. 13 - Is it possible for a molecule with the formula...Ch. 13 - Is it possible for a molecule with the formula...Ch. 13 - Is it possible for a molecule with the formula...Ch. 13 - Each molecule of butyne, C4H6, has a triple bond...Ch. 13 - Each molecule of butene, C4H8, has a double bond...Ch. 13 - Prob. 24ECh. 13 - What is the difference between aromatic and...Ch. 13 - Why are all aromatic compounds unsaturated?Ch. 13 - The carbon atoms in normal hexane, C6H14, form a...Ch. 13 - Prob. 28ECh. 13 - When sugar undergoes fermentation to produce...Ch. 13 - To what class of organic compounds does the...Ch. 13 - To what class of organic compounds does the...Ch. 13 - What have the compounds in each of these pairs in...Ch. 13 - What have the compounds in each of these pairs in...Ch. 13 - Which of the following (a) dissolve in water, (b)...Ch. 13 - Compare the properties of a simple ester, for...Ch. 13 - Why do you think the compound whose structure is...Ch. 13 - Prob. 37ECh. 13 - Use structural formulas to show the reaction...Ch. 13 - Give structural formulas for the two isomeric...Ch. 13 - Prob. 40ECh. 13 - The structural formula of acetone is shown in...Ch. 13 - Use structural formulas to show the reaction...Ch. 13 - (a) Give structural formulas for the three isomers...Ch. 13 - Prob. 44ECh. 13 - How does a plant obtain its carbohydrates and...Ch. 13 - What are the products of the oxidation of glucose?...Ch. 13 - What is believed to be the origin of atmospheric...Ch. 13 - The ultimate source of the energy in food is the...Ch. 13 - Why are unsaturated fats liquid at room...Ch. 13 - Why do plants need nitrogen? Why can they not use...Ch. 13 - What are the basic structural units of proteins?...Ch. 13 - How many letters are there in the genetic code by...Ch. 13 - What change in a gene is involved in a mutation?...Ch. 13 - To which class of organic compounds do most of the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- No chatgpt pls will upvotearrow_forwardYou are standing a distance x = 1.75 m away from this mirror. The object you are looking at is y = 0.29 m from the mirror. The angle of incidence is θ = 30°. What is the exact distance from you to the image?arrow_forwardFor each of the actions depicted below, a magnet and/or metal loop moves with velocity v→ (v→ is constant and has the same magnitude in all parts). Determine whether a current is induced in the metal loop. If so, indicate the direction of the current in the loop, either clockwise or counterclockwise when seen from the right of the loop. The axis of the magnet is lined up with the center of the loop. For the action depicted in (Figure 5), indicate the direction of the induced current in the loop (clockwise, counterclockwise or zero, when seen from the right of the loop). I know that the current is clockwise, I just dont understand why. Please fully explain why it's clockwise, Thank youarrow_forward
- A planar double pendulum consists of two point masses \[m_1 = 1.00~\mathrm{kg}, \qquad m_2 = 1.00~\mathrm{kg}\]connected by massless, rigid rods of lengths \[L_1 = 1.00~\mathrm{m}, \qquad L_2 = 1.20~\mathrm{m}.\]The upper rod is hinged to a fixed pivot; gravity acts vertically downward with\[g = 9.81~\mathrm{m\,s^{-2}}.\]Define the generalized coordinates \(\theta_1,\theta_2\) as the angles each rod makes with thedownward vertical (positive anticlockwise, measured in radians unless stated otherwise).At \(t=0\) the system is released from rest with \[\theta_1(0)=120^{\circ}, \qquad\theta_2(0)=-10^{\circ}, \qquad\dot{\theta}_1(0)=\dot{\theta}_2(0)=0 .\]Using the exact nonlinear equations of motion (no small-angle or planar-pendulumapproximations) and assuming the rods never stretch or slip, determine the angle\(\theta_2\) at the instant\[t = 10.0~\mathrm{s}.\]Give the result in degrees, in the interval \((-180^{\circ},180^{\circ}]\).arrow_forwardWhat are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V) ammeter I =arrow_forwardsimple diagram to illustrate the setup for each law- coulombs law and biot savart lawarrow_forward
- A circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.arrow_forwardAn L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forwardA long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forward
- Describe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forwardDiscuss the differences between the Biot-Savart law and Coulomb’s law in terms of their applicationsand the physical quantities they describe.arrow_forwardExplain why Ampere’s law can be used to find the magnetic field inside a solenoid but not outside.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
 Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning
Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning



Stars and Galaxies (MindTap Course List)
Physics
ISBN:9781337399944
Author:Michael A. Seeds
Publisher:Cengage Learning


Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY