
EBK THINKING LIKE AN ENGINEER
4th Edition
ISBN: 8220103633512
Author: OHLAND
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 2ICA
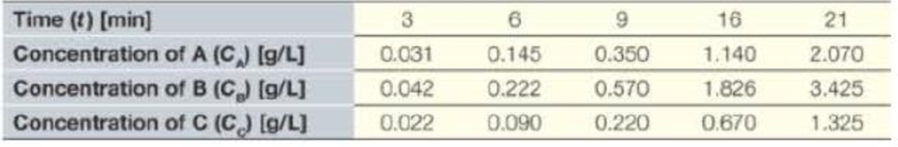
Several reactions are carried out in a closed vessel. The following data are taken for the concentration (C) of compounds A, B, and C [grams per liter] as a function of time (t) [minutes], from the start of the reaction. Show the resulting data and trendlines with equation and R2 value, on the appropriate graph type (rectilinear, semilog, or log–log) to make the data appear linear.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3-141
(3-113)
I just want to know the units of C_dot. Would it be rad/sec?
Chapter 13 Solutions
EBK THINKING LIKE AN ENGINEER
Ch. 13.2 - An unknown amount of oxygen, kept in 8 piston-type...Ch. 13.2 - The data shown graphically in the figure describe...Ch. 13.5 - Prob. 3CCCh. 13.5 - Prob. 4CCCh. 13 - Capillary action draws liquid up a narrow tube...Ch. 13 - Several reactions are carried out in a closed...Ch. 13 - An environmental engineer has obtained a bacteria...Ch. 13 - In a turbine a device used for mixing the power...Ch. 13 - Being quite interested in obsolete electronics,...Ch. 13 - Referring to the previous ICA 13-5, Angus is also...
Ch. 13 - Prob. 7ICACh. 13 - The following instructions apply to ICA 13-7 to...Ch. 13 - The following instructions apply to ICA 13-7 to...Ch. 13 - The following instructions will apply to ICA 13-10...Ch. 13 - The following instructions will apply to ICA 13-10...Ch. 13 - The following instructions will apply to ICA 13-10...Ch. 13 - The following instructions will apply to ICA 13-10...Ch. 13 - The following instructions will apply to ICA 13-10...Ch. 13 - The following instructions will apply to ICA 13-10...Ch. 13 - The following instructions will apply to ICA 13-10...Ch. 13 - The following instructions will apply to ICA 13-10...Ch. 13 - The following instructions will apply to ICA 13-10...Ch. 13 - Prob. 21ICACh. 13 - As a reminder, the Reynolds number is discussed in...Ch. 13 - As a reminder, the Reynolds number is discussed in...Ch. 13 - An environmental engineer has obtained a bacteria...Ch. 13 - An environmental engineer has obtained a bacteria...Ch. 13 - An environmental engineer has obtained a bacteria...Ch. 13 - A growing field of inquiry that poses both great...Ch. 13 - If an object is heated, the temperature of the...Ch. 13 - The Volcanic Explosivity Index (VEI) is based...Ch. 13 - You are an engineer for a plastics manufacturing...Ch. 13 - A Pitot tube is a device used to measure the...Ch. 13 - As part of an electronic music synthesizer you...Ch. 13 - The following data were collected during testing...Ch. 13 - The relationship of the power required by a...Ch. 13 - When a fluid flows around an object, it creates a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- (read image)arrow_forwardQu 2 Schematically plot attractive, repulsive, and net energies versus interatomic separation for two atoms or ions. Note on this plot the equilibrium separation (distance) ro and the bonding energy Eo. Qu 3 How many atoms (or molecules) are in one mole of the substance? Qu 4 Mole, in the context of this book, is taken in units of gram-mole. On this basis, how many atoms are there in a pound-mole of a substance? Qu 5 The atomic radii of Mg* and F ions are 0.072 and 0.133 nm, respectively. Calculate the force of attraction between these two ions at their equilibrium interionic separation (i.e., when the ions just touch one another). What is the force of repulsion at this same separation distance?show all work step by step problems formulaarrow_forwardQu 4 Silver has FCC crystal structure at room temperature, and a lattice constant, a, of 0.407 nm. Draw a reduced sphere silver unit cell in the grids provided below, clearly label the lattice dimensions. Within the unit cell you drew, shade the (1 0 0) plane. How many atoms are contained within the (1 0 0) plane? Calculate the area of (1 0 0) plane in [nm?]. Express your answer in [nm?] to three significant figures. Calculate the planar density of the (1 0 0) plane in [atoms/nm?]. Express the answer in atoms/nm to three significant figures. show all work step by steparrow_forward
- Can I get help on this question?arrow_forwardDuring some actual expansion and compression processes in piston–cylinder devices, the gases have been observed to satisfy the relationship PVn = C, where n and C are constants. Calculate the work done when a gas expands from 350 kPa and 0.03 m3 to a final volume of 0.2 m3 for the case of n = 1.5. The work done in this case is kJ.arrow_forwardCarbon dioxide contained in a piston–cylinder device is compressed from 0.3 to 0.1 m3. During the process, the pressure and volume are related by P = aV–2, where a = 6 kPa·m6. Calculate the work done on carbon dioxide during this process. The work done on carbon dioxide during this process is kJ.arrow_forward
- The volume of 1 kg of helium in a piston–cylinder device is initially 5 m3. Now helium is compressed to 3 m3 while its pressure is maintained constant at 130 kPa. Determine the initial and final temperatures of helium as well as the work required to compress it, in kJ. The gas constant of helium is R = 2.0769 kJ/kg·K. The initial temperature of helium is K. The final temperature of helium is K. The work required to compress helium is kJ.arrow_forwardA piston-cylinder device initially contains 0.4 kg of nitrogen gas at 160 kPa and 140°C. Nitrogen is now expanded isothermally to a pressure of 80 kPa. Determine the boundary work done during this process. The properties of nitrogen are R= 0.2968 kJ/kg-K and k= 1.4. N₂ 160 kPa 140°C The boundary work done during this process is KJ.arrow_forward! Required information An abrasive cutoff wheel has a diameter of 5 in, is 1/16 in thick, and has a 3/4-in bore. The wheel weighs 4.80 oz and runs at 11,700 rev/min. The wheel material is isotropic, with a Poisson's ratio of 0.20, and has an ultimate strength of 12 kpsi. Choose the correct equation from the following options: Multiple Choice о σmax= (314) (4r2 — r²) - о σmax = p² (3+) (4r² + r²) 16 σmax = (314) (4r² + r²) σmax = (314) (4² - r²)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Dynamics - Lesson 1: Introduction and Constant Acceleration Equations; Author: Jeff Hanson;https://www.youtube.com/watch?v=7aMiZ3b0Ieg;License: Standard YouTube License, CC-BY