
Concept explainers
(a)
Interpretation:
The electron-pair geometry for the molecules,
Concept introduction:
The electron pairs in Lewis diagrams repel each other in real molecule and thus they distribute themselves in positions around the central atoms that are as far away from one another. This arrangement of electron pairs is called electron-pair geometry. The electron pairs may be shared in covalent bond, or they may be lone pairs.
Answer to Problem 22E
The Lewis diagrams for
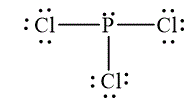 and
and 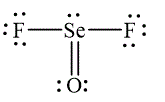
The wedge-and-dash diagrams for
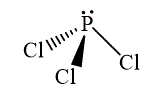 and
and 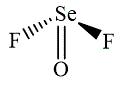
The electron pair geometry for both molecules is tetrahedral.
Explanation of Solution
To write the Lewis diagram for a compound first the number of valence electrons is to be calculated. In the molecule,
Similarly, in the molecule,
The atom which is least electronegative is the central atom. In
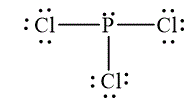
Figure 1
In
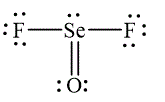
Figure 2
The electron-pair geometry depends on the number of electron pairs around the central atoms. In both the molecules
The wedge-and-dash diagram for the molecules
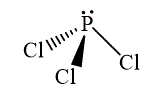
Figure 3
The wedge-and-dash diagram for the molecules

Figure 4
The Lewis and wedge-and-dash diagrams for
(b)
Interpretation:
The molecular geometry prdicted by the valence shell electron-pair repulsion theory for the molecules
Concept introduction:
Molecular geometry is the precise term that is used to describe the shape of molecules and arrangement of atoms around the central atom. The molecular geometry of a molecule is predicted by valence shell electron-pair repulsion theory or in short VSEPR theory. VSEPR theory applies to substances in which a second period element is bonded to two, three, four, or other atoms.
Answer to Problem 22E
The Lewis diagrams for
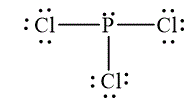 and
and 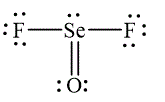
The wedge-and-dash diagrams for
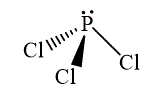 and
and 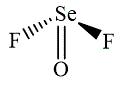
The molecular geometry for both molecules is trygonal pyramidal.
Explanation of Solution
To write the Lewis diagram for a compound first the number of valence electrons is to be calculated. In the molecule,
Similarly, in the molecule,
The atom which is least electronegative is the central atom. In
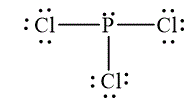
Figure 1
In
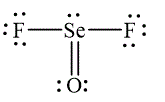
Figure 2
The molecular geometry depends on the number of electron pairs around the central atoms and the number of lone pairs present on the central atom. In the both of the molecules,
The wedge-and-dash diagram for the molecules
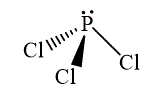
Figure 3
The wedge-and-dash diagram for the molecules
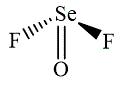
Figure 4
The Lewis and wedge-and-dash diagrams for
Want to see more full solutions like this?
Chapter 13 Solutions
EBK INTRODUCTORY CHEMISTRY: AN ACTIVE L
- Synthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forward
- Indicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forward
- Question 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward
- 2,2-Dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol. Indicate the products obtained.arrow_forwardAdd conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward3. Provide all the steps and reagents for this synthesis. OHarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
