
Pearson eText for Biochemistry: Concepts and Connections -- Instant Access (Pearson+)
2nd Edition
ISBN: 9780137533114
Author: Dean Appling, Spencer Anthony-Cahill
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 1P
Interpretation Introduction
Interpretation: The specific carbon atom eliminating as carbon dioxide from each of the glucose
Concept introduction:
Pictorial representation:
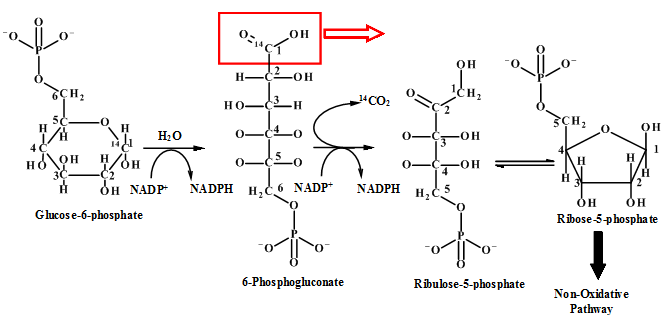
Figure 1: Pentose phosphate pathway
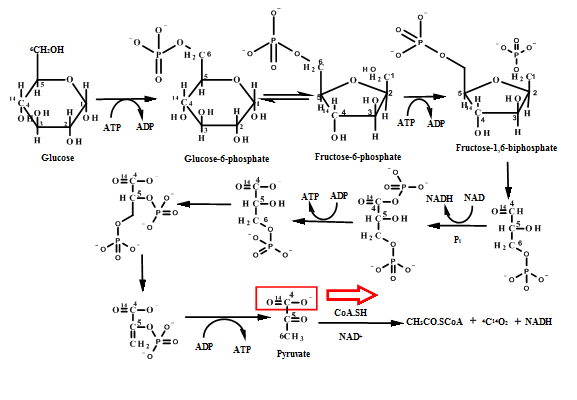
Figure 2: Glycolysis pathway
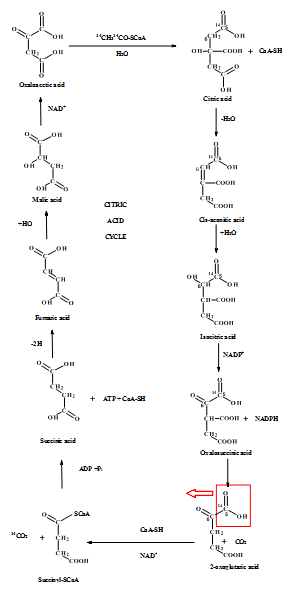
Figure 3: Citric acid cycle
Expert Solution & Answer
Explanation of Solution
From the pictorial representation it can be seen that in pentose phosphate pathway the
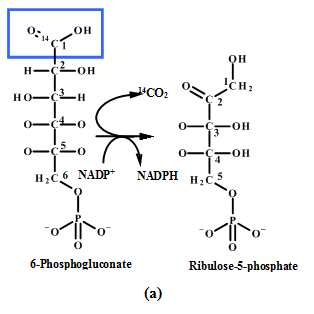 (a) | 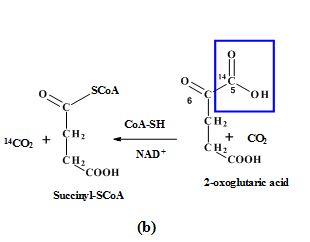 (b) |
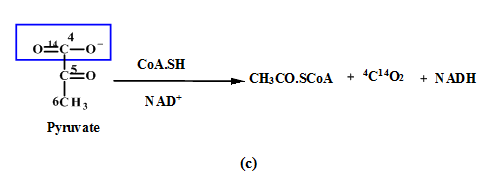 (c) | |
Figure: 4 The Thus, the proportion of glucose metabolism can be determined by the radioactive determination and estimating the amount of radioactive carbon dioxide eliminating in each step of the different pathways. The isotope of carbon i.e. 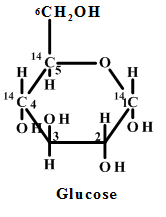 |
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
The two half reactions
for beginning and end of the electron transport chain are given below in standard form. Calculate & for the overall process. Using the Nernst equation (AG° = -n Fo, F= 96.485 kJ/volt mol), calculate AG°. Explain the need for a stepwise process in the electron transport chain.
NAD* + H+ + 2 e- = NADH
½ 0г + 2H+ + 2е- = H20
= -0.32v
E = +0.82V
answer the questions and the example steps should be from carbohydrates glycolysis and citric acid cycle. Please put down reactions and structures
identify the general type of reaction catalyzed and an example step from glycolisis structure for each of the following
enzymes/ co factor Kinase, isomerase, mutase, dehydrogenase, NAD+ , FAD
Chapter 13 Solutions
Pearson eText for Biochemistry: Concepts and Connections -- Instant Access (Pearson+)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- fill in the blanks with the missing structures and give namesarrow_forwardfill in the table and identify the general type of reaction catalayzed and an example step from the structures in the second page so you will answer the questions from the first page the second one is just a reference urgently!arrow_forwardPlease draw out the molecular structures of each molecule and show how each enzyme + cofactor would affect the following molecule in the human metabolic pathway. (This is a metabolic map)arrow_forward
- Please draw out the molecular structures of each molecule and show how an enzyme + cofactor would affect the following molecule in the human metabolic pathway to create energy.arrow_forwardPlease draw out the molecular structures of each molecule and show how each enzyme + cofactor would affect the following molecule in the human metabolic pathway.arrow_forwardPlease draw out the mechanism with curved arrows showing electron flow. Pyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvate dehydrogenase complex, resulting in acetyl-CoA and CO2. Provide the mechanism for this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forward
- Pyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvate dehydrogenase complex, resulting in acetyl-CoA and CO2. Provide the mechanism for this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forwardThe mitochondrial ATP synthase has 10 copies of the F0 subunit “c”, and the [H ] in the mitochondrial inner membrane space (IMS) is 6.31 x 10-8 M and the [H + ] in the matrix is 3.16 x 10-9 M. Calculate the minimum membrane potential (∆Ψ) necessary to make ATP synthesis thermodynamically favorable. [Assume ∆G' ofphosphate hydrolysis of ATP is - 45 kJ/mol.]arrow_forwardB- Vitamins are converted readily into important metabolic cofactors. Deficiency in any one of them has serious side effects. a. The disease beriberi results from a vitamin B 1 (Thiamine) deficiency and is characterized by cardiac and neurological symptoms. One key diagnostic for this disease is an increased level of pyruvate and α-ketoglutarate in the bloodstream. How does this vitamin deficiency lead to increased serumlevels of these factors? b. What would you expect the effect on the TCA intermediates for a patient suffering from vitamin B 5 deficiency? c. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 2 /B 3 deficiency?arrow_forward
- Pyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvate dehydrogenase complex, resulting in acetyl-CoA and CO2. Provide a full mechanism for this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forwardMap out all of the metabolic pathways in the liver cell. Draw out the structures and names of all compounds neatly by hand and the pathways responsible for metabolizing them. Some examples are: Glycolysis/gluconeogenesis, PPP, Glycogenesis/glycogenolysis, Krebs, ETC, selectamino acid pathways (Ala, Glu, Asp) Lipogenesis/lipolysis. Citrate/MAS/glycerol phosphate shuttlesystems, and the Cori/Glc-Ala cycles. Rules:-Draw both a mitochondrial area of metabolism and a cytoplasmic area of metabolism.-Draw the liver and its roles in glucose recycling (Cori cycle/Glc-Alanine recycling)-Avoid drawing the same molecule twice (except for separate mitochondrial/cytoplasmic populations. i.e. Design the PPP/Glycolysis so that GAP is only drawn once)-Label Carbon 4 of glucose and highlight where you would expect to find it in EVERY compound in whichit is present.-Have one or two locations for NADH/NADPH/ATP/GTP/CoQH2 – many arrows will come to/from thesespots.arrow_forwarda. Draw the Krebs Cycle and show the entry points for the amino acids Alanine,Glutamic Acid, Asparagine, and Valine into the Krebs Cycle. (Include name of Enzymes involved) b. How many rounds of Krebs will be required to waste all Carbons of Glutamic Acid as CO2? (Show by drawing out the mechanism that occurs)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Mitochondrial mutations; Author: Useful Genetics;https://www.youtube.com/watch?v=GvgXe-3RJeU;License: CC-BY