
Concept explainers
Freezing temperature. Ethylene glycol and propylene glycol are liquids used in antifreeze and deicing solutions. Ethylene glycol is listed as a hazardous chemical by the Environmental Protection Agency, while propylene glycol is generally regarded as safe. Table 11 lists the freezing temperature for various concentrations (as a percentage of total weight) of each chemical in a solution used to deice airplanes. A linear regression model for the ethylene glycol data in Table 11 is
where
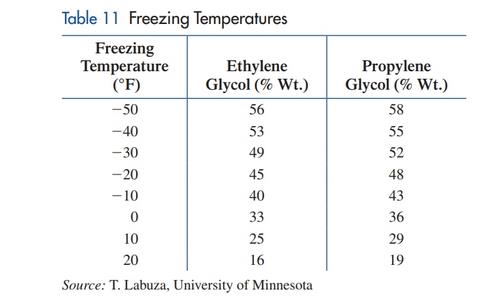
(A) Draw a
(B) Use the model to estimate the freezing temperature to the nearest degree of a solution that
is 30% ethylene glycol.
(C) Use the model to estimate the percentage of ethylene glycol in a solution that freezes at
15°F.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Pearson eText for Finite Mathematics for Business, Economics, Life Sciences, and Social Sciences -- Instant Access (Pearson+)
Additional Math Textbook Solutions
Elementary Statistics
Basic Business Statistics, Student Value Edition
Introductory Statistics
Elementary Statistics (13th Edition)
Algebra and Trigonometry (6th Edition)
- N Page 0.6. 0.4. 0.2- -0.2- -0.4- -6.6 -5 W 10arrow_forwardDiagonalize the following matrix, if possible. 8 0 6 - 8 Select the correct choice below and, if necessary, fill in the answer box to complete your choice. 8 0 OA. For P= D= 0 3 6 0 B. For P = D= 0 -6 8 0 C. For P = D= 0 - 8 D. The matrix cannot be diagonalized.arrow_forwardCalculus lll May I please have the solutions for the following exercises? Thank youarrow_forward
- 4 In the integral dxf1dy (7)², make the change of variables x = ½(r− s), y = ½(r + s), and evaluate the integral. Hint: Find the limits on r and s by sketching the area of integration in the (x, y) plane along with the r and s axes, and then show that the same area can be covered by s from 0 to r and r from 0 to 1.arrow_forward7. What are all values of 0, for 0≤0<2л, where 2 sin² 0=-sin? - 5π 6 π (A) 0, л, and 6 7π (B) 0,л, 11π , and 6 6 π 3π π (C) 5π 2 2 3 , and π 3π 2π (D) 2' 2'3 , and 3 4元 3 1 די } I -2m 3 1 -3 บ 1 # 1 I 3# 3m 8. The graph of g is shown above. Which of the following is an expression for g(x)? (A) 1+ tan(x) (B) 1-tan (x) (C) 1-tan (2x) (D) 1-tan + X - 9. The function j is given by j(x)=2(sin x)(cos x)-cos x. Solve j(x) = 0 for values of x in the interval Quiz A: Topic 3.10 Trigonometric Equations and Inequalities Created by Bryan Passwaterarrow_forwardnot use ai pleasearrow_forward
 Functions and Change: A Modeling Approach to Coll...AlgebraISBN:9781337111348Author:Bruce Crauder, Benny Evans, Alan NoellPublisher:Cengage Learning
Functions and Change: A Modeling Approach to Coll...AlgebraISBN:9781337111348Author:Bruce Crauder, Benny Evans, Alan NoellPublisher:Cengage Learning Algebra and Trigonometry (MindTap Course List)AlgebraISBN:9781305071742Author:James Stewart, Lothar Redlin, Saleem WatsonPublisher:Cengage Learning
Algebra and Trigonometry (MindTap Course List)AlgebraISBN:9781305071742Author:James Stewart, Lothar Redlin, Saleem WatsonPublisher:Cengage Learning

