
(a)
Interpretation:
For the given proposed transforms, it is to be determined whether it leads to a synthetic trap (i.e. will not proceed as planned in the forward direction) or not along with the reasoning.
Concept introduction:
A particular reaction is undone by performing a transform that depends on the specific location in the target molecule where we want the changes to occur. In doing so, we may encounter synthetic traps. A synthetic trap is the proposed mechanism that prevents the reaction to occur in the forward direction as planned. From the reactions used for
Answer to Problem 13.6P
The synthesis will proceed as planned. The forward reaction is shown below:
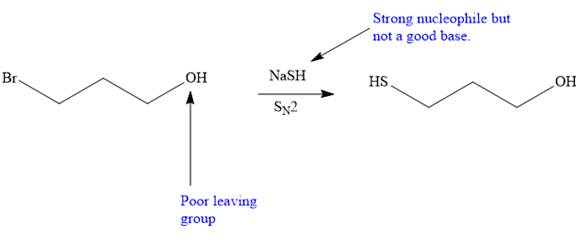
Explanation of Solution
The given proposed transform is:
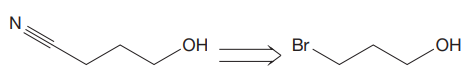
The target molecule is on the left side. In the target molecule, there are two functional groups present, a cyanide and hydroxyl group. In order to carry out this transform, sodium cyanide (

Thus, the proposed transform does not lead to a synthetic trap and will proceed as planned.
The possibility of the forward reaction in the proposed mechanism is determined on the basis of the functional group transformation reactions, charge stability, and strength of the reagents used.
(b)
Interpretation:
For the given proposed transforms, it is to be determined whether it leads to a synthetic trap (i.e. will not proceed as planned in the forward direction) or not along with the reasoning.
Concept introduction:
A particular reaction is undone by performing a transform that depends on the specific location in the target molecule where we want the changes to occur. In doing so, we may encounter synthetic traps. A synthetic trap is the proposed mechanism that prevents the reaction to occur in the forward direction as planned. From the reactions used for functional group transformation and considering the factors like charge stability and strength of the reagent used, one can determine whether the proposed transform leads to a synthetic trap or not.
Answer to Problem 13.6P
The synthesis will proceed as planned. The forward reaction is shown below:
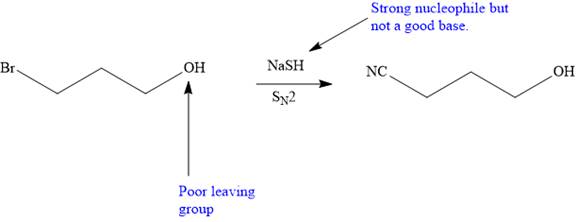
Explanation of Solution
The given proposed transform is:
![]()
The target molecule is on the left side. In the target molecule, there are two functional groups present,
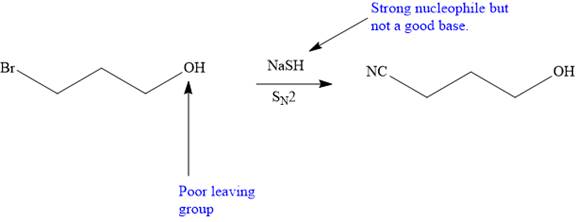
Thus, the proposed transform does not lead to a synthetic trap and will proceed as planned.
The possibility of the forward reaction in the proposed mechanism is determined on the basis of the functional group transformation reactions, charge stability, and strength of the reagents used.
(c)
Interpretation:
For the given proposed transforms, it is to be determined whether it leads to a synthetic trap (i.e. will not proceed as planned in the forward direction) or not along with the reasoning.
Concept introduction:
A particular reaction is undone by performing a transform that depends on the specific location in the target molecule where we want the changes to occur. In doing so, we may encounter synthetic traps. A synthetic trap is the proposed mechanism that prevents the reaction to occur in the forward direction as planned. From the reactions used for functional group transformation, and considering the factors like charge stability and strength of the reagent used, one can determine whether the proposed transform leads to a synthetic trap or not.
Answer to Problem 13.6P
The synthesis will proceed as planned. The forward reaction is shown below:
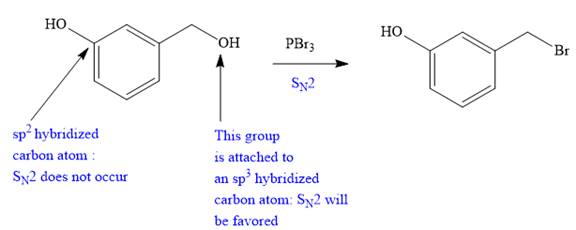
Explanation of Solution
The given proposed transform is:
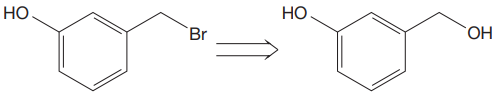
The target molecule is on the left side. In the target molecule, there are two functional groups present, alkyl halide and hydroxyl group.

Thus, the proposed transform does not lead to a synthetic trap and will proceed as planned.
The possibility of the forward reaction in the proposed mechanism is determined on the basis of the functional group transformation reactions, charge stability, and strength of the reagents used.
(d)
Interpretation:
For the given proposed transforms, it is to be determined whether it leads to a synthetic trap (i.e. will not proceed as planned in the forward direction) or not along with the reasoning.
Concept introduction:
A particular reaction is undone by performing a transform that depends on the specific location in the target molecule where we want the changes to occur. In doing so, we may encounter synthetic traps. A synthetic trap is the proposed mechanism that prevents the reaction to occur in the forward direction as planned. From the reactions used for functional group transformation, and considering the factors like charge stability and strength of the reagent used, one can determine whether the proposed transform leads to the synthetic trap or not.
Answer to Problem 13.6P
The synthesis will not proceed as planned.
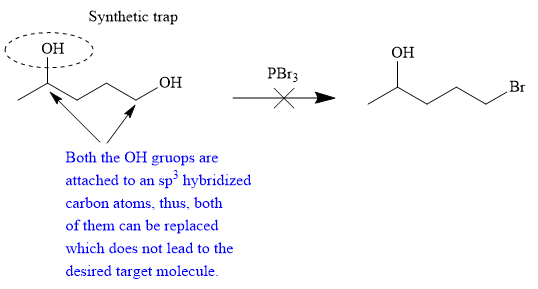
The proposed synthesis is a synthetic trap, and the reaction will not proceed as planned in the forward direction.
Explanation of Solution
The given proposed transform is:
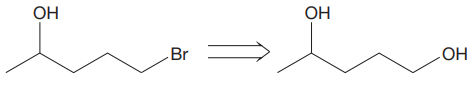
The target molecule is on the left side while the proposed reactant molecule is on the right side. In the target molecule, there are two functional groups present, alcohol and alkyl halide. Alkyl halides are prepared from corresponding alcohols when they are treated with phosphorous bromide/chloride in an

The possibility of the forward reaction in the proposed mechanism is determined on the basis of the functional group transformation reactions, charge stability, and strength of the reagents used.
(e)
Interpretation:
For the given proposed transforms, it is to be determined whether it leads to a synthetic trap (i.e. will not proceed as planned in the forward direction) or not along with the reasoning.
Concept introduction:
A particular reaction is undone by performing a transform that depends on the specific location in the target molecule where we want the changes to occur. In doing so, we may encounter synthetic traps. A synthetic trap is the proposed mechanism that prevents the reaction to occur in the forward direction as planned. From the reactions used for functional group transformation, and considering the factors like charge stability and strength of the reagent used, one can determine whether the proposed transform leads to the synthetic trap or not.
Answer to Problem 13.6P
The synthesis will not proceed as planned.
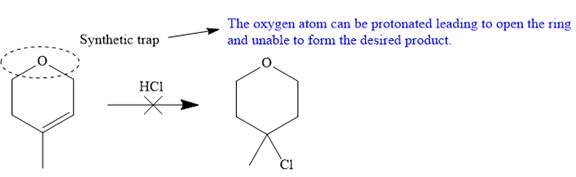
The proposed synthesis is a synthetic trap, and the reaction will not proceed as planned in the forward direction.
Explanation of Solution
The given proposed transform is:
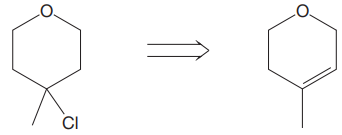
The target molecule is on the left side while the proposed reactant molecule is on the right side. In the target molecule, there are two functional groups present, ether and alkyl halide. Alkyl halides are prepared from
As the reaction does not yield the desired product, we would say that the proposed synthesis is a synthetic trap, and the reaction will not proceed as planned in the forward direction.

The possibility of the forward reaction in the proposed mechanism is determined on the basis of the functional group transformation reactions, charge stability, and strength of the reagents used.
(f)
Interpretation:
For the given proposed transforms it is to be determined whether it leads to a synthetic trap (i.e. will not proceed as planned in the forward direction) or not along with the reasoning.
Concept introduction:
A particular reaction is undone by performing a transform that depends on the specific location in the target molecule where we want the changes to occur. In doing so, we may encounter synthetic traps. A synthetic trap is the proposed mechanism that prevents the reaction to occur in the forward direction as planned. From the reactions used for functional group transformation, and considering the factors like charge stability and strength of the reagent used, one can determine whether the proposed transform leads to the synthetic trap or not.
Answer to Problem 13.6P
The synthesis will not proceed as planned.
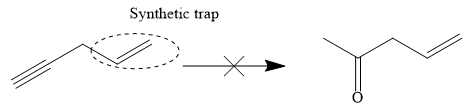
The proposed synthesis is a synthetic trap, and the reaction will not proceed as planned in the forward direction.
Explanation of Solution
The given proposed transform is:
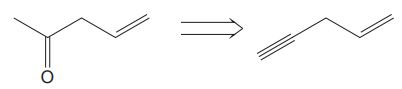
The target molecule is on the left side while the proposed reactant molecule is on the right side. In the target molecule, there are two functional groups present,
As the reaction does not yield the desired product, we would say that, the proposed synthesis is a synthetic trap and the reaction will not proceed as planned in the forward direction.

The possibility of the forward reaction in the proposed mechanism is determined on the basis of the functional group transformation reactions, charge stability, and strength of the reagents used.
Want to see more full solutions like this?
Chapter 13 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Use diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward15) Create Lewis structure Br Brarrow_forward
- LIOT S How would you make 200. mL of a 0.5 M solution of CuSO4 5H2O from solid copper (II) sulfate? View Rubricarrow_forwardSteps and explantions pleasearrow_forwardMatch the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forward
- Can you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
