
Concept explainers
(a)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates. For E2 elimination reactions, a strong base is used in the presence of a
Answer to Problem 13.33P
The solvent for the proposed synthetic step is appropriate. The proposed synthetic step is an example of an E2 reaction, which favors the Zaitsev product (most substituted alkene). If the solvent ethanol is deprotonated, the result is still an ethoxide ion which is the same base that is already shown.
Explanation of Solution
The given proposed synthetic step is:
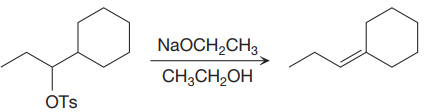
The reactant molecule has a good leaving group,
The requirement of the solvent for E2 reactions is aprotic, as aprotic solvents do not solvate the anions (negatively charged ions) as strongly as they solvate cations (positively charged ions).
(b)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates. For E2 elimination reactions, a strong base is used in the presence of a polar aprotic solvent to yield the most substituted alkene is the major product. The requirement of the solvent for E2 reactions is aprotic, as aprotic solvents do not solvate the anions (negatively charged ions) as strongly as they solvate cations (positively charged ions). If the base used in the reaction and the base produced by the deprotonation of solvent molecules is the same, then the reaction proceeds in the forward direction and the proposed synthetic step is valid and acceptable. If the base used in the reaction is different from the base produced by the deprotonation of solvent molecules, then they would compete and the proposed synthetic route then would not be the valid route.
Answer to Problem 13.33P
The solvent ethanol for the proposed synthetic step is not appropriate. The proposed synthetic step is an example of an E2 reaction, which favors the Zaitsev product (most substituted alkene). The alkoxide ion shown
Explanation of Solution
The given proposed synthetic step is:
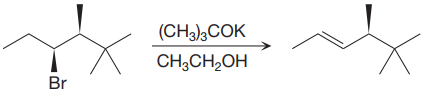
The reactant molecule has a good leaving group,
The requirement of the solvent for E2 reactions is aprotic, as aprotic solvents do not solvate the anions (negatively charged ions) as strongly as they solvate cations (positively charged ions).
(c)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates.
For a substitution reaction which follows
Answer to Problem 13.33P
The solvent ethanol for the proposed synthetic step is not appropriate. The proposed synthetic step is an example of a
Explanation of Solution
The given proposed synthetic step is:
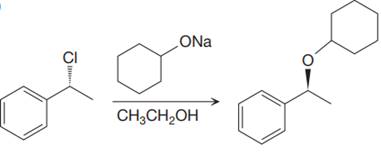
The reactant molecule has a moderate leaving group,
A
Thus, the solvent ethanol will interfere in the proposed synthetic step. Instead of ethanol cyclohexanol should be used, which will generate the same anion that is cyclohexanolate ion or any other aprotic solvent such as DMSO could be used.
The requirement of the solvent for
(d)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates.
For a substitution reaction which follows
Answer to Problem 13.33P
The solvent for the proposed synthetic step is appropriate. The proposed synthetic step is an example of an E2 reaction, which favors the Zaitsev product (most substituted alkene). If the solvent ethanol is deprotonated, the result is still an ethoxide ion which is the same base that is already shown.
Explanation of Solution
The given proposed synthetic step is:
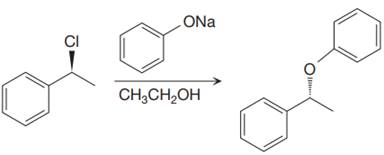
The reactant molecule has a good leaving group,
The requirement of the solvent for
Want to see more full solutions like this?
Chapter 13 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- For questions 1-4, consider the following complexes: [Co(CN)6], [COC14]², [Cr(H2O)6]²+ 4. Room temperature (20°C) measurement of molar magnetic susceptibility (Xm) for Fe(NH4)2(SO4)2×6H2O is 1.1888 x 102 cgs (Gaussian units). Calculate effective magnetic moment and provide a number of unpaired electrons for the iron ion. Use this number to rationalize the coordination geometry around iron center. (4 points)arrow_forward7. Describe the expected 31P and 19F (where applicable) NMR spectral patterns for the following compounds (indicate number of signals and their splitting patterns). a) tetraphenyldiphosphine Ph Ph P-P Ph Ph Ph Ph ' b) tetraphenyldiphosphine monoxide P-P-Ph Ph (2 points) (2 points c) tetrafluorophosphonium hexafluorophosphate [PF4]*[PF6]¯ (4 points)arrow_forward3. For questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ Which (if any) of these complexes would be expected to display Jahn-Teller distortion? (2 points)arrow_forward
- What is Instrumental Neutron Activation and what are the advantages and disadvantages in using its applications? (I'm doing an in class assignment and need better understanding of what the instrument can be used for) Please include references so that I can better understand the application of how the instrument works!arrow_forwardWhat is Isotope Analysis and what are the advantages and disadvantages in using its applications and instrumentalization? Please include references so that I can better understand how the instrument works!arrow_forward5. Count the electrons on the following complexes and state whether they follow the 18- electron rule: (3 points) Fe(CO)5 Ni(PMe3)4 PMe3 is trimethylphosphine Mn(CO)5Brarrow_forward
- For questions 1-4, consider the following complexes: [Co(CN)6]+, [CoCl4]², [Cr(H2O)6]²+ 2. Draw the corresponding d-orbital splitting for each of the complexes; predict the spin- state (low-spin/high spin) for each of the complexes (if applicable); explain your arguments. Calculate the crystal field stabilization energy for each complex (in Ao or At). (6 points)arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ 1. Assign oxidation number to the metal, then indicate d-electron count. (3 points)arrow_forwardUsing iodometry I want to titrate a sodium thiosulfate solution and I use 15 mL. If I have 50 mL of a 0.90 M copper solution and KI, what will be the molarity of sodium thiosulfate?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
