
Concept explainers
(a)
Interpretation:
An
Concept introduction:
E2 stands for bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the
Answer to Problem 13.35P
An alkyl halide that could have been used to synthesize the known alkene exclusively via an E2 reaction paying attention to stereochemistry is:
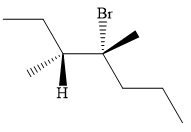
Explanation of Solution
The structure of the desired alkene is:
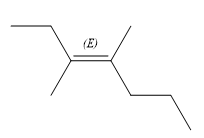
The given alkene has both the higher priority groups attached on the opposite side of the double bond. Hence, the stereochemistry is E. Alkenes can be prepared from corresponding alkyl halides via E2 reactions. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion. Thus, the original alkyl halide that could have been used to prepare the given alkene must be:

To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
(b)
Interpretation:
An alkyl halide, that can be used to synthesize the given alkene exclusively via an E2 reaction paying attention to stereochemistry, is to be provided.
Concept introduction:
The name E2 represents bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the rate of reaction, this is a bimolecular reaction. A strong base is used to form the most substituted alkene as the major product.
Answer to Problem 13.35P
An alkyl halide that could have been used to prepare the given alkene exclusively via an E2 reaction paying attention to stereochemistry is:
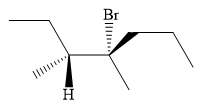
Explanation of Solution
The structure of the required alkene is:
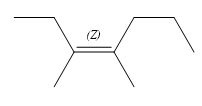
The given alkene has both the most priority groups attached on the same side of the double bond. Therefore, the stereochemistry about the double bond is Z. Alkenes can be prepared from corresponding alkyl halides via E2 reactions. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion. Thus, the original alkyl halide that could have been used to prepare the given alkene must be:

To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
(c)
Interpretation:
An alkyl halide, that can be used to synthesize the given alkene exclusively via an E2 reaction paying attention to stereochemistry, is to be provided.
Concept introduction:
The name E2 represents bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the rate of reaction, this is a bimolecular reaction. A strong base is used to form the most substituted alkene as the major product.
Answer to Problem 13.35P
An alkyl halide that could have been used to prepare the given alkene exclusively via an E2 reaction paying attention to stereochemistry is:
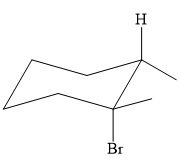
Explanation of Solution
The structure of the desired alkene is:
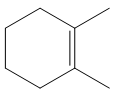
The alkene is a cycloalkene having two methyl groups attached to the double-bonded carbon atoms. As the carbon atoms in alkenes are

To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
(d)
Interpretation:
An alkyl halide, that can be used to synthesize the given alkene exclusively via an E2 reaction paying attention to stereochemistry, is to be provided.
Concept introduction:
The name E2 represents bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the rate of reaction, this is a bimolecular reaction. A strong base is used to form the most substituted alkene as the major product.
Answer to Problem 13.35P
An alkyl halide that could have been used to prepare the given alkene exclusively via an E2 reaction paying attention to stereochemistry is:
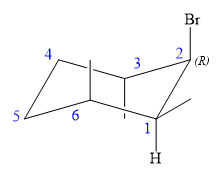
Explanation of Solution
The structure of the desired alkene is:
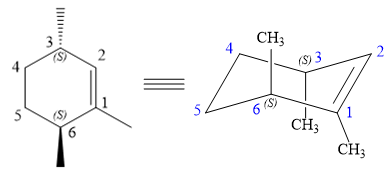
The alkene is cyclohexene having three methyl groups as substituents.
As the carbon atoms in alkenes are sp2 hybridized, all the atoms that are directly attached to the double-bonded carbon atoms must be in the plane. There are two chiral centers in the molecule at C3 and C6 carbon atoms. The stereochemistry for those two chiral centers will not change and will be retained as the reaction does not occur at those two chiral centers. Alkenes can be prepared from corresponding alkyl halides via E2 reactions.
This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion. Thus, the original alkyl halide that could have been used to prepare the given alkene must be:
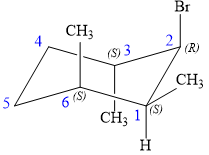
To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
(e)
Interpretation:
An alkyl halide, that can be used to synthesize the given alkene exclusively via an E2 reaction paying attention to stereochemistry, is to be provided.
Concept introduction:
The name E2 represents bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the rate of reaction, this is a bimolecular reaction. A strong base is used to form the most substituted alkene as the major product.
Answer to Problem 13.35P
An alkyl halide that could have been used to prepare the given alkene exclusively via an E2 reaction paying attention to stereochemistry is:
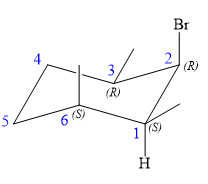
Explanation of Solution
The structure of the desired alkene is:
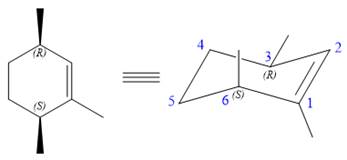
The alkene is cyclohexene having three methyl groups as substituents.
As the carbon atoms in alkenes are sp2 hybridized, all the atoms that are directly attached to the double-bonded carbon atoms must be in the plane. There are two chiral centers in the molecule at C3 and C6 carbon atoms. The stereochemistry for those two chiral centers will not change and will be retained as the reaction does not occur at those two chiral centers. Alkenes can be prepared from corresponding alkyl halides via E2 reactions.
This reaction involves a one-step mechanism (concerted) in which carbon-halogen bond and carbon-hydrogen bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion. Thus, the original alkyl halide that could have been used to synthesize the given alkene must be:
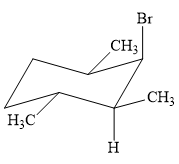
To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Want to see more full solutions like this?
Chapter 13 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forwardDraw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forward
- 3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward6. Design the most efficient synthesis of the following product starting from phenot Provide the reaction conditions for each step (more than one step is required) and explain the selectivity of each reaction. NO MECHANISMS ARE REQUIRED. OH step(s) CIarrow_forward
- What is the skeletal structure of the product of the following organic reaction?arrow_forwardIf a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWhat is the major organic product of the following nucleophilic acyl substitution reaction of an acid chloride below?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

