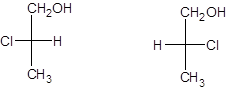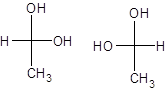CHEMISTRY: AN INTRODUCTION TO GENERAL, O
13th Edition
ISBN: 9780137444298
Author: Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 13.63APP
Interpretation Introduction
To determine: From the below given pair of Fischer’s projection formula, identify whether they are enantiomers or identical compounds.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
EEZE
LETCHUP
ID
Draw the most likely conjugate base resulting from this acid-base reaction.
Include all lone pairs. Ignore inorganic byproducts.
Drawing
く
NaOCH2CH3
:0:
:0:
狗
Answer
2. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton
transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted
without ambiguity.
a.
CH3
Ph
OEt
هد
Ph
CH3
Hint: the species on the left is an ynolate, which behaves a lot like an enolate.
Chapter 13 Solutions
CHEMISTRY: AN INTRODUCTION TO GENERAL, O
Ch. 13.1 - Prob. 13.1PPCh. 13.1 - Prob. 13.2PPCh. 13.1 - Prob. 13.3PPCh. 13.1 - Prob. 13.4PPCh. 13.1 - Prob. 13.5PPCh. 13.1 - Prob. 13.6PPCh. 13.1 - Prob. 13.7PPCh. 13.1 - Prob. 13.8PPCh. 13.1 - Prob. 13.9PPCh. 13.1 - Prob. 13.10PP
Ch. 13.2 - Prob. 13.11PPCh. 13.2 - Prob. 13.12PPCh. 13.2 - Prob. 13.13PPCh. 13.2 - Prob. 13.14PPCh. 13.2 - Prob. 13.15PPCh. 13.2 - Prob. 13.16PPCh. 13.2 - Identify each of the following as D or L: a. b. c.Ch. 13.2 - Identify each of the following as D or L: a. b. c.Ch. 13.2 - Identify the chiral carbon in each of the...Ch. 13.2 - Prob. 13.20PPCh. 13.3 - Prob. 13.21PPCh. 13.3 - Prob. 13.22PPCh. 13.3 - Prob. 13.23PPCh. 13.3 - Prob. 13.24PPCh. 13.3 - Prob. 13.25PPCh. 13.3 - Prob. 13.26PPCh. 13.3 - Prob. 13.27PPCh. 13.3 - Prob. 13.28PPCh. 13.3 - Prob. 13.29PPCh. 13.3 - Prob. 13.30PPCh. 13.4 - What are the kind and number of atoms in the ring...Ch. 13.4 - What are the kind and number of atoms in the ring...Ch. 13.4 - Prob. 13.33PPCh. 13.4 - Prob. 13.34PPCh. 13.4 - Prob. 13.35PPCh. 13.4 - Identify each of the following as the a or ß...Ch. 13.5 - Prob. 13.37PPCh. 13.5 - Prob. 13.38PPCh. 13.5 - Prob. 13.39PPCh. 13.5 - Prob. 13.40PPCh. 13.6 - Prob. 13.41PPCh. 13.6 - Prob. 13.42PPCh. 13.6 - Prob. 13.43PPCh. 13.6 - Prob. 13.44PPCh. 13.6 - Prob. 13.45PPCh. 13.6 - Prob. 13.46PPCh. 13.7 - Describe the similarities and differences in the...Ch. 13.7 - Prob. 13.48PPCh. 13.7 - Prob. 13.49PPCh. 13.7 - Prob. 13.50PPCh. 13.7 - Prob. 13.51PPCh. 13.7 - Prob. 13.52PPCh. 13.7 - Prob. 13.53PPCh. 13.7 - Prob. 13.54PPCh. 13 - Prob. 13.55UTCCh. 13 - Prob. 13.56UTCCh. 13 - Prob. 13.57UTCCh. 13 - Prob. 13.58UTCCh. 13 - Prob. 13.59UTCCh. 13 - Prob. 13.60UTCCh. 13 - Prob. 13.61APPCh. 13 - Prob. 13.62APPCh. 13 - Prob. 13.63APPCh. 13 - Prob. 13.64APPCh. 13 - Prob. 13.65APPCh. 13 - Prob. 13.66APPCh. 13 - Prob. 13.67APPCh. 13 - Prob. 13.68APPCh. 13 - Prob. 13.69APPCh. 13 - Prob. 13.70APPCh. 13 - Prob. 13.71APPCh. 13 - Prob. 13.72APPCh. 13 - Prob. 13.73APPCh. 13 - Prob. 13.74APPCh. 13 - Prob. 13.75CPCh. 13 - Prob. 13.76CPCh. 13 - 13.77 Gentiobiose is found in saffron. (13.4,...Ch. 13 - Prob. 13.78CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- b. CH3 H3C CH3 CH3 H3C an unexpected product, containing a single 9- membered ring the expected product, containing two fused rings H3C-I (H3C)2CuLi an enolatearrow_forwardb. H3C CH3 1. 2. H3O+ H3C MgBr H3Carrow_forwardPredict the major products of this reaction: excess H+ NaOH ? A Note that the first reactant is used in excess, that is, there is much more of the first reactant than the second. If there won't be any products, just check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privarrow_forward
- 1. For each of the reaction "railroads" below, you are either asked to give the structure(s) of the starting material(s) or product(s), or provide reagents/conditions to accomplish the transformation, as indicated by the boxes. a. NaOMe H+ .CO,H HO₂C MeOH (excess) MeOH H3C Br يع CH3 1. LiAlH4 2. H3O+ 3. PBг3 H3C 1. Et-Li 2. H3O+ -CO₂Me -CO₂Me OH CH3 CH3 ল CH3arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe ག1, ད།་, - + H You can draw 1 and 2 in any arrangement you like. 2 work up Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Parrow_forwardWhat is the total energy cost associated with the compound below adopting the shown conformation? CH3 HH DH CH3arrow_forward
- ΗΝ, Draw Final Product C cyclohexanone pH 4-5 Edit Enamine H3O+ CH3CH2Br THF, reflux H Edit Iminium Ionarrow_forwardHow many hydrogen atoms are connected to the indicated carbon atom?arrow_forwardIdentify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forward
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY