
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
9th Edition
ISBN: 9780136781776
Author: Wade
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.53SP
Each of these four structures has molecular formula C4cH8O2. Match the structure with its characteristic proton NMR signals. (Not all of the signals are listed in each case.)
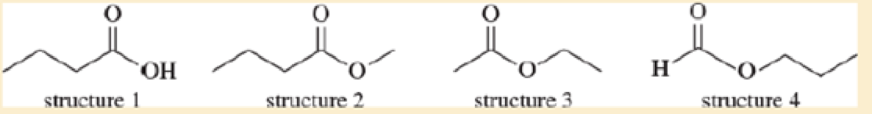
- a. sharp 1H singlet at δ 8.0 and 2H triplet at δ 4.0
- b. sharp 3H singlet at δ 2.0 and 2H quartet at δ 4.1
- c. sharp 3H singlet at δ 3.7 and 2H quartet at δ 2.3
- d. broad 1H singlet at δ 11.5 and 2H triplet at δ 2.3
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete combustion of a 0.6250 g sample of the unknown crystal with excess O2 produced 1.8546 g of CO2 and 0.5243 g of H2O. A separate analysis of a 0.8500 g sample of the blue crystal was found to produce 0.0465 g NH3. The molar mass of the substance was found to be about 310 g/mol. What is the molecular formula of the unknown crystal?
4. C6H100
5
I peak
3
2
PPM
Integration values: 1.79ppm (2), 4.43ppm (1.33)
Ipeak
None
Chapter 13 Solutions
EP ORGANIC CHEMISTRY -MOD.MASTERING 18W
Ch. 13.5A - In a 300-MHz spectrometer, the protons in...Ch. 13.5B - Prob. 13.2PCh. 13.6 - Determine the number of different kinds of protons...Ch. 13.6 - Prob. 13.4PCh. 13.7 - Draw the integral trace expected for the NMR...Ch. 13.7 - Prob. 13.6PCh. 13.8C - Draw the NMR spectra you would expect for the...Ch. 13.8D - Draw the NMR spectra you expect for the following...Ch. 13.8D - a. Assign protons to the peaks in the NMR spectrum...Ch. 13.8D - Prob. 13.10P
Ch. 13.8D - Two spectra are shown. Propose a structure that...Ch. 13.9 - Prob. 13.12PCh. 13.9 - The spectrum of trans-hex-2-enoic acid follows. a....Ch. 13.9 - Prob. 13.14PCh. 13.9 - Prob. 13.15PCh. 13.10 - Prob. 13.16PCh. 13.10 - If the imaginary replacement of either of two...Ch. 13.10 - Predict the theoretical number of different NMR...Ch. 13.11B - Prob. 13.19PCh. 13.11B - Prob. 13.20PCh. 13.11B - Prob. 13.21PCh. 13.11B - Prob. 13.22PCh. 13.11B - Prob. 13.23PCh. 13.11B - Prob. 13.24PCh. 13.12E - Draw the expected broadband-decoupled 13 C N M R...Ch. 13.12E - a. Show which carbon atoms correspond with which...Ch. 13.12E - Repeat Problem13-25, sketching the...Ch. 13.12F - Prob. 13.28PCh. 13.13 - A bottle of allyl bromide was found to contain a...Ch. 13.13 - A laboratory student was converting cyclohexanol...Ch. 13.14 - Sets of spectra are given for two compounds. For...Ch. 13 - An unknown compound has the molecular formula C 9...Ch. 13 - Prob. 13.34SPCh. 13 - Predict the approximate chemical shifts of the...Ch. 13 - Prob. 13.36SPCh. 13 - Prob. 13.37SPCh. 13 - Prob. 13.38SPCh. 13 - Prob. 13.39SPCh. 13 - Prob. 13.40SPCh. 13 - For each compound shown below. 1. sketch the 13 C...Ch. 13 - Prob. 13.42SPCh. 13 - Prob. 13.43SPCh. 13 - Prob. 13.44SPCh. 13 - Prob. 13.45SPCh. 13 - Prob. 13.46SPCh. 13 - A compound was isolated as a minor constituent in...Ch. 13 - Prob. 13.48SPCh. 13 - The three isomers of dimethylbenzene are commonly...Ch. 13 - a. Draw all six isomers of formula C 4 H 8...Ch. 13 - Prob. 13.51SPCh. 13 - Hexamethylbenzene undergoes free-radical...Ch. 13 - Each of these four structures has molecular...Ch. 13 - Prob. 13.54SPCh. 13 - Phenyl Grignard reagent adds to 2-methylpropanal...Ch. 13 - Prob. 13.56SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Consider the compounds below and determine if they are aromatic, antiaromatic, or non-aromatic. In case of aromatic or anti-aromatic, please indicate number of I electrons in the respective systems. (Hint: 1. Not all lone pair electrons were explicitly drawn and you should be able to tell that the bonding electrons and lone pair electrons should reside in which hybridized atomic orbital 2. You should consider ring strain- flexibility and steric repulsion that facilitates adoption of aromaticity or avoidance of anti- aromaticity) H H N N: NH2 N Aromaticity (Circle) Aromatic Aromatic Aromatic Aromatic Aromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic nonaromatic nonaromatic nonaromatic nonaromatic nonaromatic aromatic TT electrons Me H Me Aromaticity (Circle) Aromatic Aromatic Aromatic Aromatic Aromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic nonaromatic nonaromatic nonaromatic nonaromatic nonaromatic aromatic πT electrons H HH…arrow_forwardA chemistry graduate student is studying the rate of this reaction: 2 HI (g) →H2(g) +12(g) She fills a reaction vessel with HI and measures its concentration as the reaction proceeds: time (minutes) [IH] 0 0.800M 1.0 0.301 M 2.0 0.185 M 3.0 0.134M 4.0 0.105 M Use this data to answer the following questions. Write the rate law for this reaction. rate = 0 Calculate the value of the rate constant k. k = Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol.arrow_forwardNonearrow_forward
- in which spectral range of EMR, atomic and ionic lines of metal liesarrow_forwardQ2: Label the following molecules as chiral or achiral, and label each stereocenter as R or S. CI CH3 CH3 NH2 C CH3 CH3 Br CH3 X &p Bra 'CH 3 "CH3 X Br CH3 Me - N OMe O DuckDuckarrow_forward1. For the four structures provided, Please answer the following questions in the table below. a. Please draw π molecular orbital diagram (use the polygon-and-circle method if appropriate) and fill electrons in each molecular orbital b. Please indicate the number of π electrons c. Please indicate if each molecule provided is anti-aromatic, aromatic, or non- aromatic TT MO diagram Number of π e- Aromaticity Evaluation (X choose one) Non-aromatic Aromatic Anti-aromatic || ||| + IVarrow_forward
- 1.3 grams of pottasium iodide is placed in 100 mL of o.11 mol/L lead nitrate solution. At room temperature, lead iodide has a Ksp of 4.4x10^-9. How many moles of precipitate will form?arrow_forwardQ3: Circle the molecules that are optically active: ДДДДarrow_forward6. How many peaks would be observed for each of the circled protons in the compounds below? 8 pts CH3 CH3 ΤΙ A. H3C-C-C-CH3 I (₁₁ +1)= 7 H CI B. H3C-C-CI H (3+1)=4 H LIH)=2 C. (CH3CH2-C-OH H D. CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY