
Concept explainers
(a)
Interpretation:
The difference in the proton NMR spectra of the given compound is to be stated.
Concept introduction:
The NMR stands for nuclear magnetic resonance. NMR spectroscopy deals with the interaction between
Answer to Problem 13.44AP
The difference in the proton NMR spectra of the compound A and compound B is that compound A contains a doublet and septet and compound B contains only singlet.
Explanation of Solution
The given compounds are shown below.
(CH3)2CH-Cl (CH3)2CD-Cl A B
The formula used to determine NMR splitting is shown below.
Number of peaks=n+1 … (1)
Where
• n is the number of hydrogen atom on the adjacent atom.
The structure of compound A is shown below.
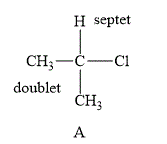
Figure 1
The compound A shown in Figure 1 contains six methyl protons and one methyne proton. The number of hydrogen atoms on adjacent atom of methyl protons (n) is 1. Substitute the value of n in equation (1) as shown below.
Number of peaks=1+1=2
Therefore, the NMR spectra of methyl protons splits into a doublet as the number of peaks is 2. On the other hand, the number of hydrogen atoms on adjacent atom of the methyne proton (n) is 6. Substitute the value of n in equation (1) as shown below.
No. of peaks=6+1=7
NMR spectra of methyne protons splits into septet as the number of peaks is 6.
The structure of the compound B is shown below.
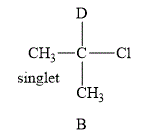
Figure 2
The compound B shown in Figure 2 contains six methyl protons. The number of hydrogen atoms on adjacent atom of methyl protons (n) is 0. Substitute the value of n in equation (1) as shown below.
Number of peaks=0+1=1
The NMR spectra of methyl protons splits into singlet as the number of peaks is 0. As a result, there is no coupling. Therefore, the methyl protons give a singlet peak.
The compound A gives septet and doublet whereas compound B gives a singlet in proton NMR spectra.
(b)
Interpretation:
The difference in the proton NMR spectra of the given compound is to be stated.
Concept introduction:
The NMR stands for nuclear magnetic resonance. NMR spectroscopy deals with the interaction between electromagnetic radiation and the nucleus of an atom. NMR spectroscopy is used to determine the structural information about compounds.
Answer to Problem 13.44AP
The difference in the proton NMR spectra of the compound A and compound B is that compound A contains two triplets and compound B contains two triplets and one quintet (multiplet).
Explanation of Solution
The given compounds are shown below.
Cl-CD2CH2CH2-Cl Cl-CH2CH2CH2-Cl A B
The formula used to determine NMR splitting is shown below.
Number of peaks=n+1 …(1)
Where
• n is the number of hydrogen atom on the adjacent atom.
The structure of compound A is shown below.
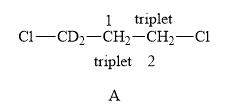
Figure 3
The compound A shown in Figure 3 contains four methylene protons. The methylene protons are adjacent to each other. Therefore, both of them couple with each other. The value of hydrogen atoms (n) on adjacent atom of C−1 is 2. Substitute the value of n in equation (1) as shown below.
Number of peaks=2+1=3
Therefore, the NMR spectra of methyl protons of C−1 splits into a triplet as the number of peaks is 3. Similarly, the value of a number of hydrogen atoms (n) on adjacent atom of C−2 is 2. Substitute the value of n in equation (1) a shown below.
Number of peaks=2+1=3
Therefore, the NMR spectra of methyl protons of C−2 splits into a triplet as the number of peaks is 3.
The structure of compound B is shown below.
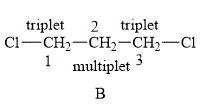
Figure 4
The compound B shown in Figure 4 contains six methylene protons. The methylene protons are adjacent to each other. The value of hydrogen atoms (n) on adjacent atom of C−1 is 2. Substitute the value of n in equation (1) as shown below.
Number of peaks=2+1=3
Therefore, the NMR spectra of methyl protons of C−1 splits into a triplet as the number of peaks is 3. Similarly, the value of a number of hydrogen atoms (n) on adjacent atom of C−3 is 2. Substitute the value of n in equation (1) a shown below.
Number of peaks=2+1=3
Therefore, the NMR spectra of methyl protons of C−3 splits into a triplet as the number of peaks is 3.
The protons on the adjacent of C−2 are chemically equivalent. The value of hydrogen atoms (n) on adjacent atom of C−2 is 4. Substitute the value of n in equation (1) as shown below.
Number of peaks=4+1=5
Therefore, the NMR spectra of methyl protons of C−2 splits into a quintet (multiplet) as the number of peaks is 5.
The compound A gives triplet at both the carbons whereas compound B gives triplet on C−1 and C−3 but proton NMR spectra.
(c)
Interpretation:
The difference in the proton NMR spectra of the given compound is to be stated.
Concept introduction:
The NMR stands for nuclear magnetic resonance. NMR spectroscopy deals with the interaction between electromagnetic radiation and the nucleus of an atom. NMR spectroscopy is used to determine the structural information about compounds.
Answer to Problem 13.44AP
The difference in the proton NMR spectra of the compound A and compound B is that compound A contains a doublet and septet and compound B contains only singlet.
Explanation of Solution
The formula used to determine NMR splitting is shown below.
Number of peaks=n+1 …(1)
Where
• n is the number of hydrogen atom on the adjacent atom.
The structure of compound A and B are shown below.
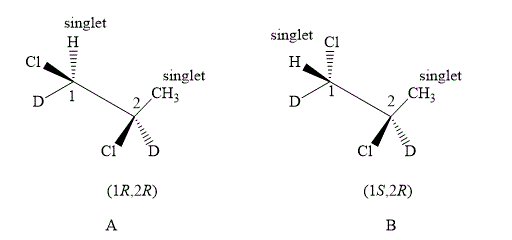
Figure 5
The compound A and B shown in Figure 5 both have similar spectrums. In the compound A, the value of number of methyl protons on adjacent to C−1 is 0. Substitute the value of n in equation (1) as shown below.
Number of peaks=0+1=1
Therefore, the NMR spectra of methyl protons of C−1 splits into a singlet as the number of peaks is 1. Similarly the value of a number of hydrogen atoms (n) on adjacent atom of C−2 is 0. Substitute the value of n in equation (1) a shown below.
Number of peaks=0+1=1
Therefore, the NMR spectra of methyl protons of C−2 splits into a singlet as the number of peaks is 1.
Compound A and compound B both are diastereotopic. The chemical shift value of both the compounds is slightly different. The spectrum of both compounds is the same as there are diastereoisomers. Therefore, compound B also forms singlet on both the carbons.
The structure of compound C is shown below.
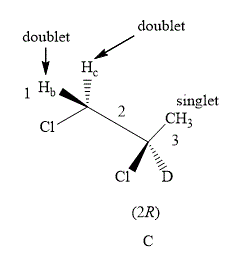
Figure 6
The compound C shown in Figure 6 contains five equivalent protons. The value of hydrogen atoms (n) on adjacent atom of C−3 is 0. Substitute the value of n in equation (1) as shown below.
Number of peaks=0+1=1
Therefore, the NMR spectra of methyl protons of C−3 splits into a singlet as the number of peaks is 1 as the neighboring protons are equivalent. Similarly the value of a number of hydrogen atoms (n) on adjacent atom of C−2 is 1. Substitute the value of n in equation (1) is shown below.
No. of peaks=1+1=2
Therefore, the NMR spectra of methyl protons of C−2 splits into a doublet as the number of peaks is 2.
The number of peaks of both C−1 and C−2 is same as both the protons are surrounded by same neighboring protons. The number of protons (n) adjacent to C−1 is 1. Substitute the value of n in equation (1) is shown below.
Number of peaks=1+1=2
Therefore, the NMR spectra of methyl protons of C−1 splits into a doublet as the number of peaks is 2.
The compound A gives triplet at both the carbons whereas compound B gives triplet on C−1 and C−3 but proton NMR spectra.
Want to see more full solutions like this?
Chapter 13 Solutions
ORGANIC CHEM +SG +SAPLING >IP<
- Indicate the substitutes in one place, if they are a diazonio room.arrow_forwardIndicate the product formed in each reaction. If the product exhibits tautomerism, draw the tautomeric structure. a) о + CH3-NH-NH2 CO2C2H5 b) + CoH5-NH-NH2 OC2H5arrow_forwardIndicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forward
- Synthesis of 1-metilbenzotriazole from 1,2-diaminobenceno.arrow_forwardSynthesis of 1-metilbenzotriazole.arrow_forwardIndicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forward
- Identify the mechanism through which the following reaction will proceed and draw the major product. Part 1 of 2 Br KOH EtOH Through which mechanism will the reaction proceed? Select the single best answer. E1 E2 neither Part: 1/2 Part 2 of 2 Draw the major product formed as a result of the reaction. Click and drag to start drawing a structure. Xarrow_forwardWhat is single-point calibration? Provide an example.arrow_forwardDraw the major product formed via an E1 pathway.arrow_forward
- Part 9 of 9 Consider the products for the reaction. Identify the major and minor products. HO Cl The E stereoisomer is the major product and the Z stereoisomer is the minor product ▼ S major product minor productarrow_forwardConsider the reactants below. Answer the following questions about the reaction mechanism and products. HO Clarrow_forwardjulietteyep@gmail.com X YSCU Grades for Juliette L Turner: Orc X 199 A ALEKS - Juliette Turner - Modul X A ALEKS - Juliette Turner - Modul x G butane newman projection - Gox + www-awa.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBxzaplnN4HsoQggFsejpgqKoyrQrB2dKVAN-BcZvcye0LYa6eXZ8d4vVr8Nc1GZqko5mtw-d1MkNcNzzwZsLf2Tu9_V817y?10Bw7QYjlb il Scribbr citation APA SCU email Student Portal | Main Ryker-Learning WCU-PHARM D MySCU YSCU Canvas- SCU Module 4: Homework (Ch 9-10) Question 28 of 30 (1 point) | Question Attempt: 1 of Unlimited H₂SO heat OH The mechanism of this reaction involves two carbocation intermediates, A and B. Part 1 of 2 KHSO 4 rearrangement A heat B H₂O 2 OH Draw the structure of A. Check Search #t m Save For Later Juliet Submit Assignm 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





