
Concept explainers
Interpretation:
The molecular weight of each explosive in this chemical connection should be determined along with identify which explosive do the nitro groups contribute the largest percentage of molecular weight.
Concept Introduction:
The following explosives are present in the chemical connections.
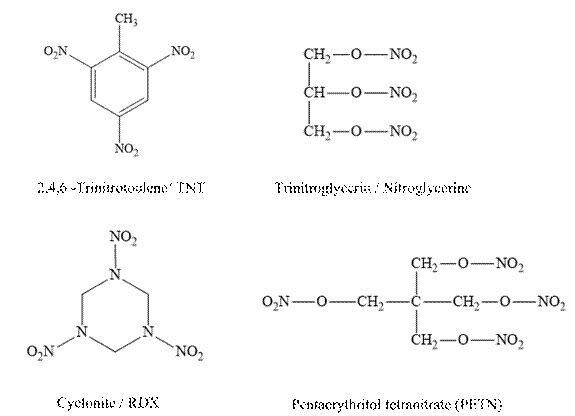
Answer to Problem 13.41P
Molecular weight of TNT is
Molecular weight of nitroglycerine is.
Molecular weight of cyclonite is.
Molecular weight of pentaerythritol is
Therefore, cyclonite has high percentage contribution of nitro group in their molecular weight.
Explanation of Solution
Molecular weight of TNT (
Atomic weight of total number of carbon atoms =
Atomic weight of total number of hydrogen atoms =
Atomic weight of total number of nitrogen atoms =
Atomic weight of total number of oxygen atoms =
Molecular weight =
Therefore, molecular weight of TNT is
Percentage composition of
TNT has three
Weight of.
Therefore, the percentage contribution of.
Percentage contribution of nitrogen in TNT:
TNT has three nitrogens.
Therefore, the percentage contribution of nitrogen in TNT is.
Molecular weight of nitroglycerine (
Atomic weight of total number of carbon atoms =
Atomic weight of total number of hydrogen atoms =
Atomic weight of total number of nitrogen atoms =
Atomic weight of total number of oxygen atoms =
Molecular weight =
Therefore, molecular weight of nitroglycerine is
Percentage composition of
Nitroglycerine has three
Weight of.
Therefore, the percentage contribution of.
Percentage contribution of nitrogen in nitroglycerine:
Nitroglycerine has three nitrogens.
Therefore, the percentage contribution of nitrogen in nitroglycerine is
Molecular weight of cyclonite (
Atomic weight of total number of carbon atoms =.
Atomic weight of total number of hydrogen atoms =.
Atomic weight of total number of nitrogen atoms =
Atomic weight of total number of oxygen atoms =
Molecular weight =
Therefore, molecular weight of cyclonite is
Percentage composition of
Cyclonite has three
Weight of
Therefore, the percentage contribution of.
Percentage contribution of nitrogen in cyclonite:
Cyclonite has six nitrogens.
Therefore, the percentage contribution of nitrogen in cyclonite is.
Molecular weight of pentaerythritol (
Atomic weight of total number of carbon atoms =
Atomic weight of total number of hydrogen atoms =
Atomic weight of total number of nitrogen atoms =
Atomic weight of total number of oxygen atoms =.
Molecular weight =
Therefore, molecular weight of pentaerythritol is
Percentage composition of
Pentaerythritol has four
Weight of
Therefore, the percentage contribution of
Percentage contribution of nitrogen in pentaerythritol
Pentaerythritol has four nitrogens.
Therefore, the percentage contribution of nitrogen in pentaerythritol is
Molecular weight of TNT is
Molecular weight of nitroglycerine is
Molecular weight of cyclonite is
Molecular weight of pentaerythritol is
Therefore, cyclonite has high percentage contribution of nitro group in their molecular weight.
Want to see more full solutions like this?
Chapter 13 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- Indicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forward
- Synthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forward
- Indicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
