
Concept explainers
(a)
Interpretation:
The structure of the compound having molecular formula,
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an
Answer to Problem 13.40AP
The structure of the compound having molecular formula,
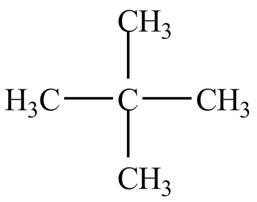
Explanation of Solution
There is only one signal in the compound that means all the protons are equivalent and all the protons are from methyl group as per chemical shift value of
Therefore, the structure of the compound corresponding to NMR data is shown below.
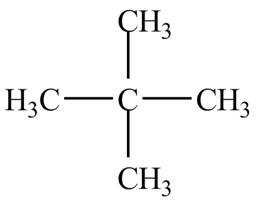
Figure 1
The structure of the compound having molecular formula,
(b)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
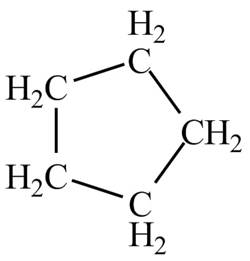
Explanation of Solution
There is only one signal in the compound that means all the protons are equivalent and all the protons are from methylene group as per chemical shift value of
Therefore, the structure of the compound corresponding to NMR data is shown below.
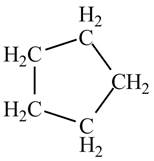
Figure 2
The structure of the compound having molecular formula
(c)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
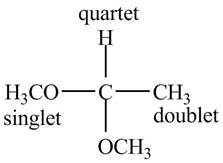
Explanation of Solution
There are three signals in the compound. One is at
Therefore, the structure of the compound corresponding to NMR data is shown below.

Figure 3
The structure of the compound having molecular formula
(d)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
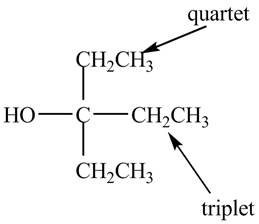
Explanation of Solution
The given spectrum is shown below.
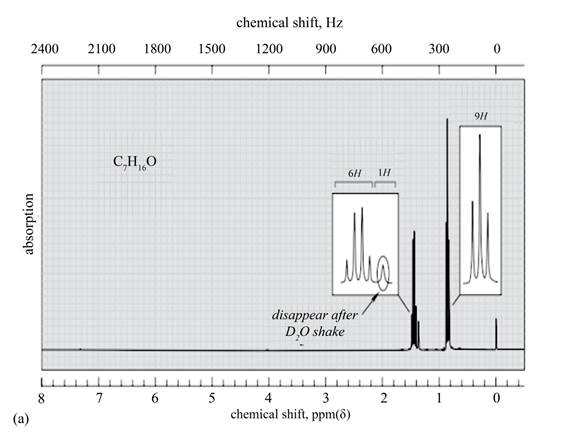
Figure 4
There are three signals in the compound. One is at
Therefore, the structure of the compound corresponding to NMR data is shown below.

Figure 5
The structure of the compound having molecular formula
(e)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
Explanation of Solution
The given spectrum is shown below.
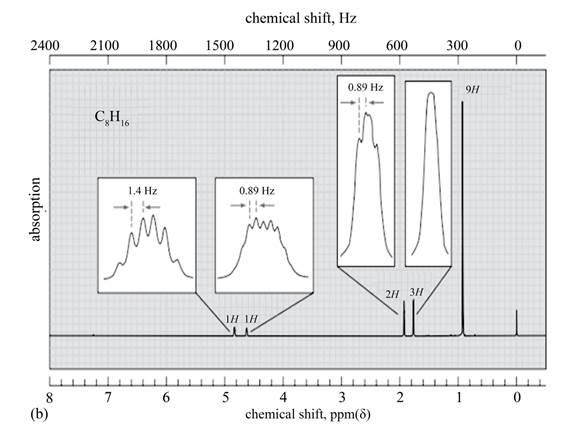
Figure 6
It is given that the
The
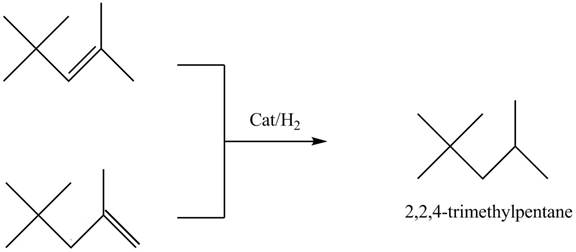
Figure 7
There are five signals in the compound. The two signal at
Therefore, the structure of the compound corresponding to NMR data is shown below.

Figure 8
The structure of the compound having molecular formula
(f)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
Explanation of Solution
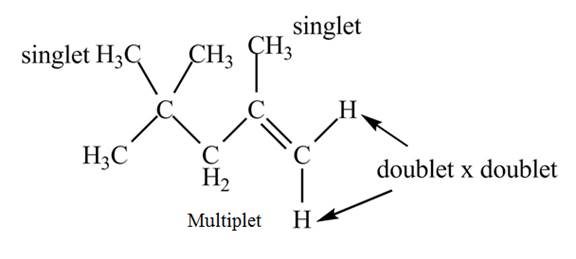
There are three signals in the compound. The signal at
Therefore, the structure of the compound corresponds to NMR data as shown below.

Figure 9
The structure of the compound having molecular formula
(g)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
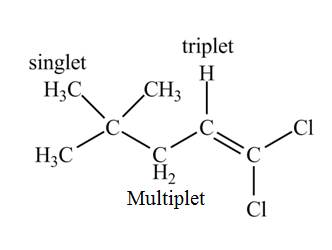
The structure of the compound having molecular formula
Explanation of Solution
There is only one signal in the compound for two hydrogens that means both are equivalent. The triplet signal is given by the two protons that means the splitting of the signal is done by two equivalent fluorine groups.
Therefore, the structure of the compound corresponding to NMR data is shown below.

Figure 10
The structure of the compound having molecular formula
(h)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
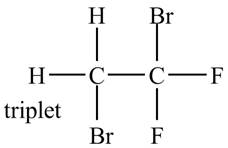
The structure of the compound having molecular formula
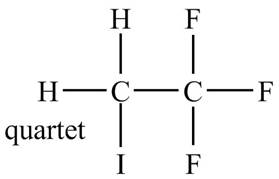
Explanation of Solution
There is only one signal in the compound for two hydrogens that means both are equivalent. The quartet signal is given by the two protons that means the splitting of the signal is done by three equivalent fluorine groups.
Therefore, the structure of the compound corresponding to NMR data is shown below.

Figure 11
The structure of the compound having molecular formula
(i)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
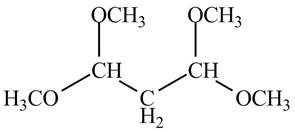
Explanation of Solution
There are three signals in the compound and total hydrogens are sixteen. The relative intergral tells about the ratio of number of hydrogen in each signal. The relative integral is
Therefore, the structure of the compound corresponding to NMR data is shown below.

Figure 12
The structure of the compound having molecular formula
(j)
Interpretation:
The structure of the compound having molecular formula
Concept introduction:
Many nuclei and electrons have spin. Due to this spin magnetic moment arises. The energy of this magnetic moment depends on the orientation of the applied magnetic field. In NMR spectroscopy, every nucleus has a spin. There is an angular momentum related to the spin. The difference between its resonance frequency and that of the reference standard is known as the chemical shift of a nucleus. Tetramethylsilane (TMS) is taken as reference.
Answer to Problem 13.40AP
The structure of the compound having molecular formula
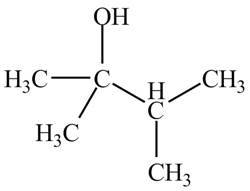
Explanation of Solution
There are three signals in the compound and total hydrogens are fourteen. The doublet signal at
The signal at
Therefore, the structure of the compound corresponding to NMR data is shown below.
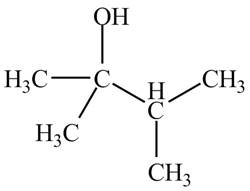
Figure 13
The structure of the compound having molecular formula
Want to see more full solutions like this?
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY
- Can I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forward
- I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

