
On December 19, 2007, the T2 Laboratories, Inc., reactor exploded in a runaway reaction. The reaction of methyl cyclopentadienyl dimer and sodium produces sodium methyl cyclopentadiene and hydrogen:
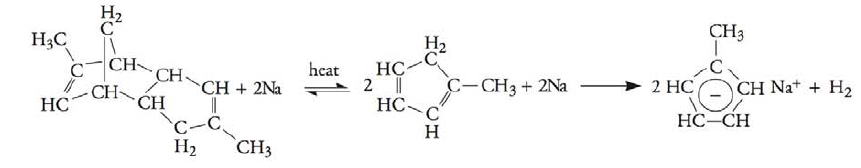
The reactor has to be cooled when its temperature reaches
If the rare constant for a typical reaction is
if all the heat were released at once?
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
EBK CHEMISTRY: THE MOLECULAR NATURE OF
Additional Science Textbook Solutions
Anatomy & Physiology (6th Edition)
Introductory Chemistry (6th Edition)
Campbell Biology (11th Edition)
Campbell Biology in Focus (2nd Edition)
Fundamentals of Physics Extended
Cosmic Perspective Fundamentals
- Silicon forms a series of compounds analogous to the al-kanes and having the general formula SinH2n+2. The first of these compounds is silane, SiH4, which is used in the electronics industry to produce thin ultrapure silicon films. SiH4(g) is somewhat difficult to work with because it is py-ropboric at room temperature—meaning that it bursts into flame spontaneously when exposed to air. (a) Write an equation for the combustion of SiH4(g). (The reaction is analogous to hydrocarbon combustion, and SiO2 is a solid under standard conditions. Assume the water produced will be a gas.) (b) Use the data from Appendix E to calculate ? for this reaction. (c) Calculate G and show that the reaction is spontaneous at 25°C. (d) Compare G for this reaction to the combustion of methane. (See the previous problem.) Are the reactions in these two exercises enthalpy or entropy driven? Explain.arrow_forwardAmoxicillin is an antibiotic packaged as a powder. When it is used to treat babies and small animals, the pharmacist or veterinarian must suspend it in water, so that it can be administered orally with a medicine dropper. The label says to dispose of unused suspension after 14 days. It also points out that refrigeration is required. In the context of this chapter, what is implied in the latter two statements?arrow_forwardSubstances that poison a catalyst pose a major concern for many engineering designs, including those for catalytic converters. One design option is to add materials that react with potential poisons before they reach the catalyst. Among the commonly encountered catalyst poisons are silicon and phosphorus, which typically form phosphate or silicate ions in the oxidizing environment of an engine. Group 2 elements are added to the catalyst to react with these contaminants before they reach the working portion of the catalytic converter. If estimates show that a catalytic converter will be exposed to 625 g of silicon during its lifetime, what mass of beryllium would need to be included in the design?arrow_forward
- Can a reaction mechanism ever be proven correct? Can it be proven incorrect?arrow_forwardPeroxynitric acid (HOONO2) is an unstable molecule that decomposes to nitric acid and oxygen: 2HOONO2(aq) → 2HNO3(aq) + O2(g)When the concentration of peroxynitic acid is graphed against time, the resulting plot is curved, but if the logarithm of this concentration is plotted, we instead get a straight line. Based on this, which statement is true? a) This decay is a second order in peroxynitric acid. b) The slope of the straight-line graph is the rate constant. c) One needs the concentration of peroxynitric acid to calculate its half-life. d) The rate law appears to be of the form -Δ[HOONO2]/Δt = k[HOONO2].arrow_forwardO to 185s? 185 to 416? 416 to 815 s? The decomposition of N,O, can be described by the equation 2 N,0,(soln) → 4 NO, (soln) + 0, (g) Consider the data in the table for the reaction at 45 °C in carbon tetrachloride solution. t (s) [N,0,] (M) 1.876 185 1.670 416 1.444 815 1.124 Given the data, calculate the average rate of reaction for each successive time interval.arrow_forward
- Scientists can determine the age of ancient objects by the method of radiocarbon dating. The bombardment of the upper atmosphere by cosmic rays converts nitrogen to a radioactive isotope of carbon, 14C, with a half-life of about 5,730 years. Vegetation absorbs carbon dioxide through the atmosphere, and animal life assimilates 14C through food chains. When a plant or animal dies, it stops replacing its carbon, and the amount of 14C present begins to decrease through radioactive decay. Therefore, the level of radioactivity must also decay exponentially. Dinosaur fossils are too old to be reliably dated using carbon-14. Suppose we had a 66 million year old dinosaur fossil. What percent of the living dinosaur's 14C would be remaining today? (Round your answer to five decimal places.) 0.0000007537 X % Suppose the minimum detectable amount is 0.2%. What is the maximum age (in years) of a fossil that we could date using 14C? (Round your answer to the nearest year.) yrarrow_forwardScientists can determine the age of ancient objects by the method of radiocarbon dating. The bombardment of the upper atmosphere by cosmic rays converts nitrogen to a radioactive isotope of carbon, 14C, with a half-life of about 5,730 years. Vegetation absorbs carbon dioxide through the atmosphere, and animal life assimilates 14C through food chains. When a plant or animal dies, it stops replacing its carbon, and the amount of 14C present begins to decrease through radioactive decay. Therefore, the level of radioactivity must also decay exponentially. a) Dinosaur fossils are too old to be reliably dated using carbon-14. Suppose we had a 67 million year old dinosaur fossil. What percent of the living dinosaur's 14C would be remaining today? (Round your answer to five decimal places.) b) Suppose the minimum detectable amount is 0.7%. What is the maximum age (in years) of a fossil that we could date using 14C? (Round your answer to the nearest year.)arrow_forwardSucrose (C12H22O11)(C12H22O11), which is commonly known as table sugar, reacts in dilute acid solutions to form two simpler sugars, glucose and fructose, both of which have the formula C6H12O6C6H12O6. At 23 ∘C∘C and in 0.5 M HClM HCl, the following data were obtained for the disappearance of sucrose: Time (min)(min) C12H22O11(M)C12H22O11(M) 0 0.316 39 0.274 80 0.238 140 0.190 210 0.146 What is the rate constant?arrow_forward
- The decomposition of N,O, can be described by the equation 2N,0, (soln) –→ 4 NO, (soln) + 0, (g) Consider the data in the table for the reaction at 45 °C in carbon tetrachloride solution. 1 (s) [N,O;] (M) 2.287 165 2.062 466 1.706 725 1.449 Given the data, calculate the average rate of reaction for each successive time interval. What is the average rate of reaction for the time interval from 0 s to 165 s? average rate of reaction: 0.00136 M/s Incorrect What is the average rate of reaction for the time interval from 165 s to 466 s?arrow_forwardHello, i need help with these questions. Please provide a handwritten solution for better understanding. One pathway for the destruction of ozone in the upper atmosphere is O3(g) + NO(g)---> NO2(g) + O2(g) Slow NO2(g) + O(g) ---> NO(g) + O2(g) Fast Overall reaction: O3(g) + O(g)---> 2O2(g) Ea is 14.0 kJ/mol, for the uncatalyzed reaction: O3(g) + O(g) --> 2O2(g) 1a) Ea for the same reaction, when catalyzed as above, is 11.9 kJ/mol.At -37°C, what is the ratio of the rate constant for the catalyzed reaction to that for the uncatalyzed reaction?(Assume the frequency factor A is the same for each reaction.) 1b) One of the concerns about the use of freons is that they will migrate to the upper atmosphere, where chlorine atoms can be generated by the reaction CCl2F2 CF2Cl + Cl (photolysis of Freon-12) Chlorine atoms can also act as a catalyst for the destruction of ozone.The activation energy for the reaction Cl + O3 --> ClO + O2 is 2.1…arrow_forwardChlorine dioxide (ClO₂) is a disinfectant used in municipal water treatment plants. It dissolves in basic solution producing ClO3 and ClO₂": 2 CLO₂(g) + 2 OH (aq) → ClO3(aq) + ClO₂ (aq) + H₂O(l) › The following kinetic data were obtained at 298 K for the reaction. Experiment [CIO₂]0 (M) [OH-]o (M) Initial Rate (M/s) 1 0.060 0.030 0.0248 2 0.020 0.030 0.00827 3 0.020 0.090 0.0247 Determine the rate law by indicating the values of "x" and "y" in the general rate law below. Rate = K[ClO₂]X[OH-]Y X = 1 = 1 y = OB b. Calculate the rate constant for this reaction at 298 K. Sig. Figs. are important. M-1. S-1 Rate constant, k = 184.4arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning




