
Concept explainers
(a)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates. For E2 elimination reactions, a strong base is used in the presence of a
Answer to Problem 13.33P
The solvent for the proposed synthetic step is appropriate. The proposed synthetic step is an example of an E2 reaction, which favors the Zaitsev product (most substituted alkene). If the solvent ethanol is deprotonated, the result is still an ethoxide ion which is the same base that is already shown.
Explanation of Solution
The given proposed synthetic step is:
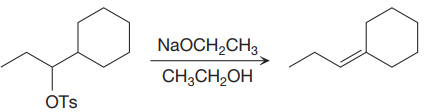
The reactant molecule has a good leaving group,
The requirement of the solvent for E2 reactions is aprotic, as aprotic solvents do not solvate the anions (negatively charged ions) as strongly as they solvate cations (positively charged ions).
(b)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates. For E2 elimination reactions, a strong base is used in the presence of a polar aprotic solvent to yield the most substituted alkene is the major product. The requirement of the solvent for E2 reactions is aprotic, as aprotic solvents do not solvate the anions (negatively charged ions) as strongly as they solvate cations (positively charged ions). If the base used in the reaction and the base produced by the deprotonation of solvent molecules is the same, then the reaction proceeds in the forward direction and the proposed synthetic step is valid and acceptable. If the base used in the reaction is different from the base produced by the deprotonation of solvent molecules, then they would compete and the proposed synthetic route then would not be the valid route.
Answer to Problem 13.33P
The solvent ethanol for the proposed synthetic step is not appropriate. The proposed synthetic step is an example of an E2 reaction, which favors the Zaitsev product (most substituted alkene). The alkoxide ion shown
Explanation of Solution
The given proposed synthetic step is:
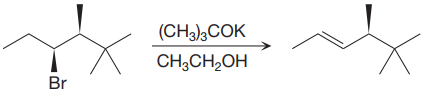
The reactant molecule has a good leaving group,
The requirement of the solvent for E2 reactions is aprotic, as aprotic solvents do not solvate the anions (negatively charged ions) as strongly as they solvate cations (positively charged ions).
(c)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates.
For a substitution reaction which follows
Answer to Problem 13.33P
The solvent ethanol for the proposed synthetic step is not appropriate. The proposed synthetic step is an example of a
Explanation of Solution
The given proposed synthetic step is:
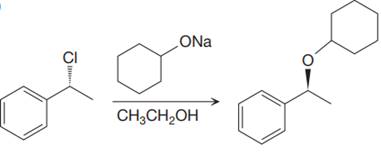
The reactant molecule has a moderate leaving group,
A
Thus, the solvent ethanol will interfere in the proposed synthetic step. Instead of ethanol cyclohexanol should be used, which will generate the same anion that is cyclohexanolate ion or any other aprotic solvent such as DMSO could be used.
The requirement of the solvent for
(d)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates.
For a substitution reaction which follows
Answer to Problem 13.33P
The solvent for the proposed synthetic step is appropriate. The proposed synthetic step is an example of an E2 reaction, which favors the Zaitsev product (most substituted alkene). If the solvent ethanol is deprotonated, the result is still an ethoxide ion which is the same base that is already shown.
Explanation of Solution
The given proposed synthetic step is:
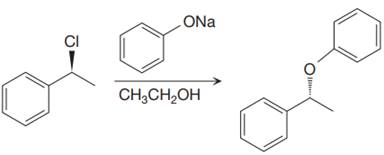
The reactant molecule has a good leaving group,
The requirement of the solvent for
Want to see more full solutions like this?
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
