
(a)
Interpretation:
The given statements have to be answered.
Concept Introduction:
The time taken by the concentration of reaction to get reduced of its original concentration is called as half-life reaction.
Half life for first order reactions:
The half life for the first order reaction is constant and it is independent of the reactant concentration.
Half life period of first order reaction can be calculated using the equation,
Half life for second order reactions:
In second order reaction, the half-life is inversely proportional to the initial concentration of the reactant (A).
The half-life of second order reaction can be calculated using the equation,
Since the reactant will be consumed in lesser amount of time, these reactions will have shorter half-life.
To complete the pictures
(a)
Explanation of Solution
The reaction follows first order with presence of half-life of ten seconds.
There are 16 AB particles present in the container,
After one half life (10s) 8 particles will be reacted and 8 remains unreacted.
After two-half lives (20s) 12 particles will be reacted and 4 remains unreacted.
The completed pictures are,
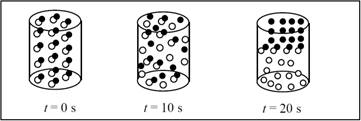
Figure 1
(b)
Interpretation:
The given statements have to be answered.
Concept Introduction:
The time taken by the concentration of reaction to get reduced of its original concentration is called as half-life reaction.
Half life for first order reactions:
The half life for the first order reaction is constant and it is independent of the reactant concentration.
Half life period of first order reaction can be calculated using the equation,
Half life for second order reactions:
In second order reaction, the half-life is inversely proportional to the initial concentration of the reactant (A).
The half-life of second order reaction can be calculated using the equation,
Since the reactant will be consumed in lesser amount of time, these reactions will have shorter half-life.
To explain the changes in completed figure if the reaction was second-order with same half life
(b)
Explanation of Solution
If the half-life is similar for second-order reaction, the container t=20s would have more number of AB and fewer A and B when compared to part a.
(c)
Interpretation:
The given statements have to be answered.
Concept Introduction:
The time taken by the concentration of reaction to get reduced of its original concentration is called as half-life reaction.
Half life for first order reactions:
The half life for the first order reaction is constant and it is independent of the reactant concentration.
Half life period of first order reaction can be calculated using the equation,
Half life for second order reactions:
In second order reaction, the half-life is inversely proportional to the initial concentration of the reactant (A).
The half-life of second order reaction can be calculated using the equation,
Since the reactant will be consumed in lesser amount of time, these reactions will have shorter half-life.
To give the relative
(c)
Explanation of Solution
After 10 seconds, the concentration of the particles is one-half their initial value. Then relative rate of reactions for first-order at the start and after 10 seconds are,
(d)
Interpretation:
The given statements have to be answered.
Concept Introduction:
The time taken by the concentration of reaction to get reduced of its original concentration is called as half-life reaction.
Half life for first order reactions:
The half life for the first order reaction is constant and it is independent of the reactant concentration.
Half life period of first order reaction can be calculated using the equation,
Half life for second order reactions:
In second order reaction, the half-life is inversely proportional to the initial concentration of the reactant (A).
The half-life of second order reaction can be calculated using the equation,
Since the reactant will be consumed in lesser amount of time, these reactions will have shorter half-life.
To give the relative reaction rates for second order reaction at the start of reaction and after 10 seconds elapsed
(d)
Explanation of Solution
After 10 seconds, the concentration of the particles is one-half their initial value. Then relative rate of reactions for second order at the start and after 10 seconds are,
Want to see more full solutions like this?
Chapter 13 Solutions
Bundle: General Chemistry, Loose-leaf Version, 11th + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card
- At an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forwardExplain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.arrow_forward
- Explain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





