
Concept explainers
Interpretation:
The missing reagents for each step in Your Turn 13.6 are to be supplied.
Concept introduction:
In order to identify the missing reagents in the given reaction sequence, it is important to identify if the reaction involves a
Under basic conditions, the nucleophile attacks the
Answer to Problem 13.1P
The missing reagents for each step in the given reaction sequence are given below:
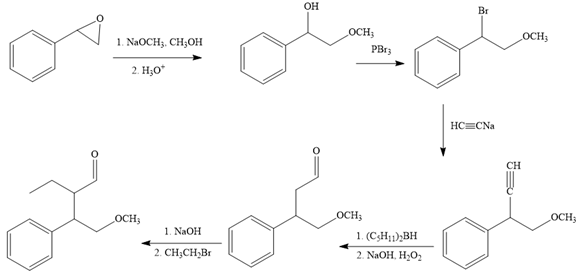
Explanation of Solution
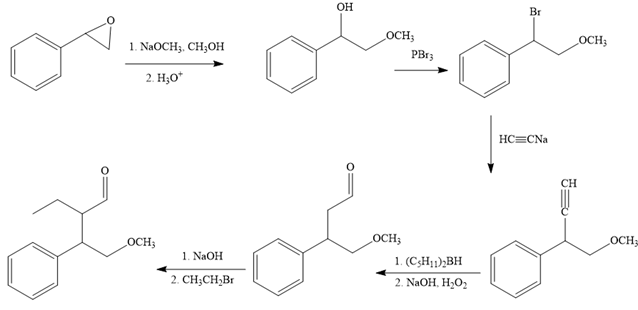
The reaction sequence given in Your Turn 13.6 is:
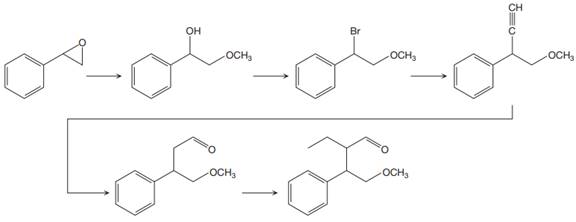
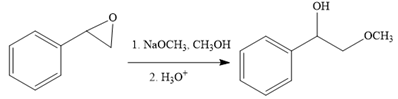
The first reaction is the conversion of an epoxide to alcohol. Thus, it is a functional group transformation reaction in which the nucleophile,
The first reaction and the missing reagents for it are shown below:
The second reaction also involves functional group transformation. The alcoholic (
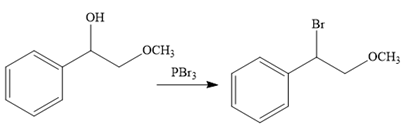
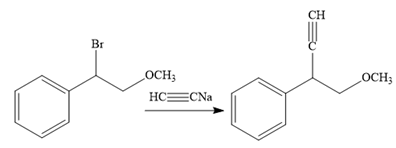
In the third reaction, the bromine atom is replaced by an acetylene group (
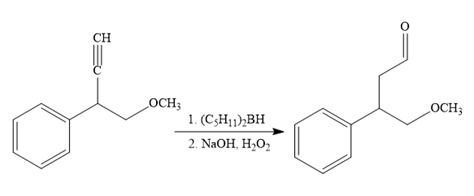
The fourth reaction in the given reaction sequence is a reaction involving a functional group transformation. Terminal alkynes undergo a hydroboration-oxidation reaction which leads to the formation of an aldehyde. The reagents used in the hydroboration-oxidation reaction are disiamylborane [
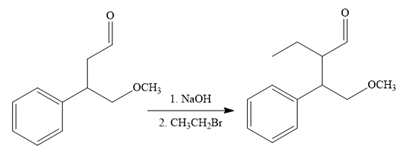
The fifth reaction involves the alteration of the carbon skeleton. The alpha hydrogen attached to an alpha carbon in aldehydes is weakly acidic. A strong base abstracts this alpha hydrogen to form an enolate ion. This enolate serves as a nucleophile and reacts with an alkyl halide via
The complete reaction sequence with appropriate reagents for each are given below:
In order to identify the missing reagents in the given reaction sequence, it is important to identify if the reaction involves a functional group transformation or it is a reaction that alters the carbon skeleton.
Want to see more full solutions like this?
Chapter 13 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- achieve.macmillanlearning.com Canvas EA eac h Hulu YouTube G 3 methyl cyclobutanol - Google Search Ranking Phenol Acidity Course -236 - Organic Chemistry - Mac... ← Assessment Completed 10 of 22 Questions 1 + Netflix paramount plus chem hw Galdehyde reaction with grignard reagent... b My Questions | bartleby M Inbox - chenteislegit@gmail.com - Gmail Due: Fri, Jan 31 Resources Solution Penalized ? Hint Submit Answer Use retrosynthetic analysis to suggest two paths to synthesize 2-methyl-3-hexanol using the Grignard reaction. (Click and drag the appropriate image to the correct position in the reactions.) Route 1 Aldehyde 1 or +98 Aldehyde 2 Route 2 Q6 +100 Solved in 1 attempt Q7 +95 Solved in 2 attempts Q8 +98 Unlimited attempts possible + + Grignard 1 OH H3O+ Grignard 2 Answer Bank Q9 +90 MgBr Unlimited attempts possible CH3CH2CH2MgBr Q10 Unlimited attempts Q11 ? ? +100 in 1 attempt 2-methyl-3-hexanol CH3CH2MgBr H H о H Attempt 3arrow_forward2) (4 pt) After the reaction was completed, the student collected the following data. Crude product data is the data collected after the reaction is finished, but before the product is purified. "Pure" product data is the data collected after attempted purification using recrystallization. Student B's data: Crude product data "Pure" product data after recrystallization Crude mass: 0.93 g grey solid Crude mp: 96-106 °C Crude % yield: Pure mass: 0.39 g white solid Pure mp: 111-113 °C Pure % yield: a) Calculate the crude and pure percent yields for the student's reaction. b) Summarize what is indicated by the crude and pure melting points.arrow_forwardDon't used hand raitingarrow_forward
- A DEPT NMR spectrum is shown for a molecule with the molecular formula of C5H12O. Draw the structure that best fits this data. 200 180 160 140 120 100 一盆 00 40 8- 20 ppm 0 Qarrow_forwardDon't used hand raitingarrow_forwardShown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. H. +N=C H H H Cl: Click and drag to start drawing a structure. : ? g B S olo Ar B Karrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
