
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.18P
What
compounds
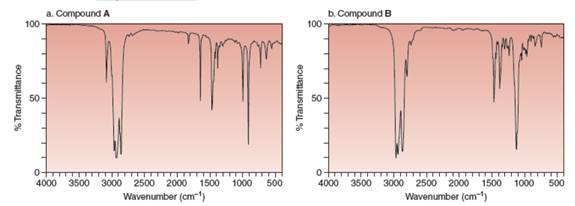
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
What is the final product when D-galactose reacts with hydroxylamine?
Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.
Chapter 13 Solutions
ORGANIC CHEMISTRY
Ch. 13 - What is the mass of the molecular ion formed from...Ch. 13 - Prob. 13.2PCh. 13 - Use the following information to propose a...Ch. 13 - Prob. 13.4PCh. 13 - What molecular ions would you expect for the...Ch. 13 - The mass spectrum of 2,3-dimethylpentane also...Ch. 13 - The base peak in the mass spectrum of 2, 2,...Ch. 13 - (a) What mass spectral fragments are formed by ...Ch. 13 - What cations are formed in the mass spectrometer...Ch. 13 - The low-resolution mass spectrum of an unknown...
Ch. 13 - Benzene, toluene, and p-xylene BTX are often added...Ch. 13 - Prob. 13.12PCh. 13 - Prob. 13.13PCh. 13 - Prob. 13.14PCh. 13 - Prob. 13.15PCh. 13 - How do the IR spectra of the isomers cyclopentane...Ch. 13 - Problem 13.17 How do the three isomers of...Ch. 13 - Problem 13.18 What functional groups are...Ch. 13 - Problem-13.19 What are the major IR absorptions in...Ch. 13 - Problem-13.20 What are the major IR absorptions in...Ch. 13 - Problem-13.21 Which of the following possible...Ch. 13 - Problem-13.22 Propose structures consistent with...Ch. 13 - 13.23 What major IR absorptions are present above ...Ch. 13 - Problem-13.24 The mass spectrum of the following...Ch. 13 - Prob. 13.25PCh. 13 - Which compound gives a molecular ion at m/z= 122,...Ch. 13 - Propose two molecular formulas for each molecular...Ch. 13 - Propose four possible structures for a hydrocarbon...Ch. 13 - Problem-13.29 What is the molecular formula for...Ch. 13 - Problem-13.30 Propose a molecular formula for rose...Ch. 13 - 13.31 Match each structure to its mass spectrum
Ch. 13 - 13.32 Propose two possible structures for a...Ch. 13 - 13.33 What cations are formed in the mass...Ch. 13 - 13.34 and have the same molecular ion in the...Ch. 13 - 13.35 For each compound, assign likely...Ch. 13 - Prob. 13.36PCh. 13 - 13.37 Propose a structure consistent with each...Ch. 13 - 13.38 A low-resolution mass spectrum of the...Ch. 13 - 13.39 Primary alcohols often show a peak in their...Ch. 13 - 13.40 Like alcohols, ethers undergo α cleavage by...Ch. 13 - 13.41 Which of the highlighted bonds absorbs at...Ch. 13 - 13.42 What major IR absorptions are present above ...Ch. 13 - 13.43 How would each of the following pairs of...Ch. 13 - 13.44 Morphine, heroin, and oxycodone are three...Ch. 13 - 13.45 Reduction of cyclohex-2-enone can yield...Ch. 13 - Prob. 13.46PCh. 13 - 13.47 Match each compound to its IR spectrum
Ch. 13 - 13.48 Propose possible structures consistent with...Ch. 13 - A chiral hydrocarbon X exhibits a molecular ion at...Ch. 13 - 13.50 A chiral compound has a strong absorption...Ch. 13 - 13.51 Treatment of benzoic acid with followed by...Ch. 13 - 13.52 Treatment of benzaldehyde with in aqueous ...Ch. 13 - Prob. 13.53PCh. 13 - 13.54 Reaction of 2-methylpropanoic acid with ...Ch. 13 - 13.55 Reaction of pentanoyl chloride with lithium...Ch. 13 - Prob. 13.56PCh. 13 - 13.57 Treatment of anisole with and forms P,...Ch. 13 - 13.58 Reaction of with forms compound ,...Ch. 13 - Problem-13.59 The carbonyl absorption of an amide...Ch. 13 - Prob. 13.60PCh. 13 - Problem-13.61 Explain why a ketone carbonyl...Ch. 13 - 13.62 Oxidation of citronellol, a constituent of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
An electric motor has an effective resistance of 32.0 and an inductive reactance of 45.0 when working under l...
Fundamentals of Physics Extended
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
Why is it necessary to be in a pressurized cabin when flying at 30,000 feet?
Anatomy & Physiology (6th Edition)
1. Suppose a chloride ion and a sodium ion are separated by a center—center distance of 5 Å. Is
the interactio...
Biochemistry: Concepts and Connections (2nd Edition)
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY