
(a)
Interpretation:
The rate constant for the growth in the number of transistor on an integrated circuit has to be determined using the given plot ln N versus year.
Concept introduction:
Rate of the reaction is the change in the concentration of reactant or a product with time.
The rate law expresses the relationship of the
Rate equation for the general reaction
Order of a reaction: The sum of exponents of the concentrations in the rate law for the reaction is said to be order of a reaction.
For first order reaction,
Moore’s law states that the number of transistors per square inch on integrated circuits had doubled every year since their invention (1958).
(a)
Explanation of Solution
Given plot of ln N versus t (year) is shown below,
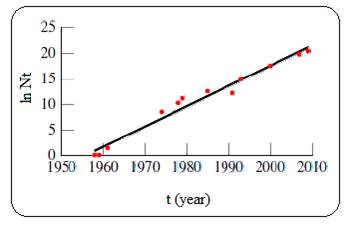
Figure 1
The plot of ln N versus t is linear for a process which follows first order kinetics. And so the given process follows first order kinetics.
The rate can be described using the equation,
Where N is the number of transistor on an integrated circuit, which is roughly doubles every 1.5 year according to the Moore’s law.
For first order reaction,
For this case, the equation can be rearranged as follows,
Comparing this equation to the straight line equation (
(b)
Interpretation:
The time required for
Concept introduction:
The rate law expresses the relationship of the rate of a reaction to the rate constant.
Rate equation for the general reaction
Order of a reaction: The sum of exponents of the concentrations in the rate law for the reaction is said to be order of a reaction.
For first order reaction,
Moore’s law states that the number of transistors per square inch on integrated circuits had doubled every year since their invention (1958).
(b)
Explanation of Solution
Given plot of ln N versus t (year) is shown below,

Figure 1
The time required for
For first order reaction,
This value is very close to the value mentioned in Moore’s law.
(c)
Interpretation:
The number of transistors on an integrated circuit
Concept introduction:
The rate law expresses the relationship of the rate of a reaction to the rate constant.
Rate equation for the general reaction
Order of a reaction: The sum of exponents of the concentrations in the rate law for the reaction is said to be order of a reaction.
For first order reaction,
Moore’s law states that the number of transistors per square inch on integrated circuits had doubled every year since their invention (1958).
(c)
Explanation of Solution
Given plot of ln N versus t (year) is shown below,

Figure 1
The time required for
For first order reaction,
For this case, the equation can be rearranged as follows,
Assume the year 1960 as
The year 2100 would corresponds to
Substituting known values in the above mentioned equation,
Thus, there will be
Want to see more full solutions like this?
Chapter 13 Solutions
Chemistry
- Q1: For each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label each compound as chiral or achiral. OH HO CI Br H CI CI Br CI CI Xf x f g Br D OH Br Br H₂N R. IN Ill I -N S OMe D II H CO₂H 1/111 DuckDuckGarrow_forwardThese are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". All of the carbon atoms of the products must come from the starting material! ? H Harrow_forwardQ5: Draw every stereoisomer for 1-bromo-2-chloro-1,2-difluorocyclopentane. Clearly show stereochemistry by drawing the wedge-and-dashed bonds. Describe the relationship between each pair of the stereoisomers you have drawn.arrow_forward
- Classify each pair of molecules according to whether or not they can participate in hydrogen bonding with one another. Participate in hydrogen bonding CH3COCH3 and CH3COCH2CH3 H2O and (CH3CH2)2CO CH3COCH3 and CH₂ CHO Answer Bank Do not participate in hydrogen bonding CH3CH2OH and HCHO CH3COCH2CH3 and CH3OHarrow_forwardNonearrow_forwardQ4: Comparing (3S,4S)-3,4-dimethylhexane and (3R,4S)-3,4-dimethylhexane, which one is optically active? Briefly explain.arrow_forward
- Nonearrow_forwardNonearrow_forwardGiven the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 4A (g) + 2B (g) → 2C (g) + 7D (g) AHrxn =?kJ Substance AH in kJ/mol A (g) - 20.42 B (g) + 32.18 C (g) - 72.51 D (g) - 17.87arrow_forward
- Determine ASran for Zn(s) + 2HCl(aq) = ZnCl2(aq) + H2(aq) given the following information: Standard Entropy Values of Various Substance Substance So (J/mol • K) 60.9 Zn(s) HCl(aq) 56.5 130.58 H2(g) Zn2+(aq) -106.5 55.10 CI (aq)arrow_forward3) Catalytic hydrogenation of the compound below produced the expected product. However, a byproduct with molecular formula C10H12O is also formed in small quantities. What is the by product?arrow_forwardWhat is the ΔHorxn of the reaction? NaOH(aq) + HCl(aq) → H2O(l) + NaCl(aq) ΔHorxn 1= ________ kJ/molarrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





