
Concept explainers
The equilibrium constant for the reaction
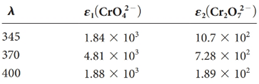
Four solutions were preparedby dissolving 4.00 × 10-4, 3.00 × 10-4, 2.00 × 10-4,and 1.00 × 10-4 moles of K2 Cr2 O7 in water and diluting to 1.00 L with a pH 5.60 buffer. Derive theoretical absorbance values (1.00-cm cells) for each solution and plot the data for (a) 345 nm, (b) 370 nm, and (c) 400 nm.
(a)
Interpretation:
The theoretical absorbance value for 345 nm should be derived and the data should be plotted.
Concept introduction:
The relationship between absorbance and concentration of absorbance is linear. If the incident light
In the case of no absorbing sample, all the light gets passed and the value of
Explanation of Solution
Given:
The equilibrium constant is
The given reaction is
The equilibrium constant for the given reaction is
The formula to determine pH is:
Therefore
For the given reaction the expression for the equilibrium constant can be written as
The equilibrium concentration of dichromate is
The theoretical absorbance value of the first solution can be calculated as below
For the second solution, everything will remain the same, just the equilibrium concentration of dichromate will change to
So, the new required concentration will be as
The theoretical absorbance value of the first solution can be calculated as below
For the third solution, everything will remain the same, just the equilibrium concentration of dichromate will change to
So, the new required concentration will be as
The theoretical absorbance value of the first solution can be calculated as below
For the fourth solution, everything will remain the same, just the equilibrium concentration of dichromate will change to
So, the new required concentration will be as
The theoretical absorbance value of the first solution can be calculated as below:
Now take the values in excel and plot to get the graph:
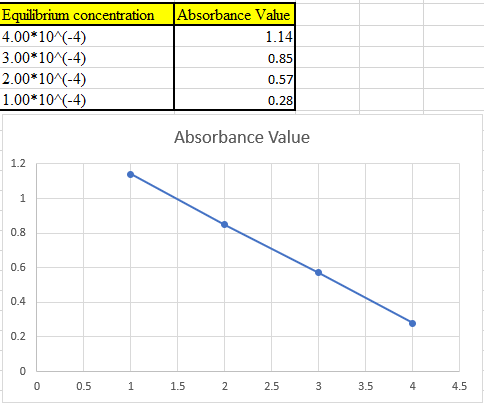
(b)
Interpretation:
The theoretical absorbance value for 370 nm should be derived and the data should be plotted.
Concept introduction:
The relationship between absorbance and concentration of absorbance is linear. If the incident light
In the case of no absorbing sample, all the light gets passed and the value of
Explanation of Solution
Given:
The equilibrium constant is
The given reaction is
The equilibrium constant for the given reaction is
The formula to determine pH is:
Therefore
For the given reaction the expression for the equilibrium constant can be written as
The equilibrium concentration of dichromate is
The theoretical absorbance value of the first solution can be calculated as below
For the second solution, everything will remain the same, just the equilibrium concentration of dichromate will change to
So, the new required concentration will be as
The theoretical absorbance value of the first solution can be calculated as below
For the third solution, everything will remain the same, just the equilibrium concentration of dichromate will change to
So, the new required concentration will be as
The theoretical absorbance value of the first solution can be calculated as below
For the fourth solution, everything will remain the same, just the equilibrium concentration of dichromate will change to
So, the new required concentration will be as
The theoretical absorbance value of the first solution can be calculated as below
Now take the values in excel and plot to get the graph:
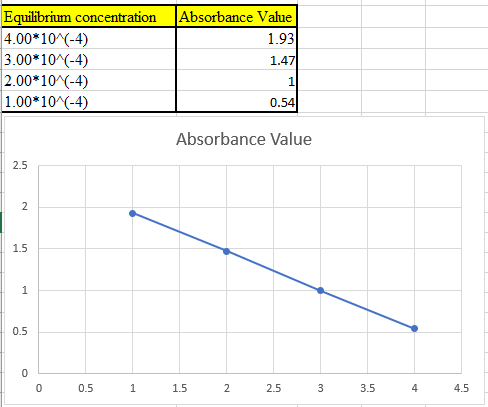
(c)
Interpretation:
The theoretical absorbance value for 400 nm should be derived and the data should be plotted.
Concept introduction:
The relationship between absorbance and concentration of absorbance is linear. If the incident light
In the case of no absorbing sample, all the light gets passed and the value of
Explanation of Solution
Given:
The equilibrium constant is
The given reaction is
The equilibrium constant for the given reaction is
The formula to determine pH is:
Therefore
For the given reaction the expression for the equilibrium constant can be written as
The equilibrium concentration of dichromate is
The theoretical absorbance value of the first solution can be calculated as below
For the second solution, everything will remain the same, just the equilibrium concentration of dichromate will change to
So, the new required concentration will be as
The theoretical absorbance value of the first solution can be calculated as below
For the third solution, everything will remain the same, just the equilibrium concentration of dichromate will change to
So, the new required concentration will be as
The theoretical absorbance value of the first solution can be calculated as below
For the fourth solution, everything will remain the same, just the equilibrium concentration of dichromate will change to
So, the new required concentration will be as
Theoretical absorbance value of first solution can be calculated as below
Now take the values in excel and plot to get the graph:
Want to see more full solutions like this?
Chapter 13 Solutions
Principles of Instrumental Analysis, 6th Edition
- What are the major products of the following reaction? Draw all the major products. If there are no major products, then there is no reaction that will take place. Use wedge and dash bonds when necessary.arrow_forwardZeolites. State their composition and structure. Give an example.arrow_forwardDon't used hand raiting and show all reactionsarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardIX) By writing the appropriate electron configurations and orbital box diagrams briefly EXPLAIN in your own words each one of the following questions: a) The bond length of the Br2 molecule is 2.28 Å, while the bond length of the compound KBr is 3.34 Å. The radius of K✶ is 1.52 Å. Determine the atomic radius in Å of the bromine atom and of the bromide ion. Br = Br b) Explain why there is a large difference in the atomic sizes or radius of the two (Br and Br). Tarrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forward
- When 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol. Which experimental number must be initialled by the Lab TA for the first run of Part 1 of the experiment? a) the heat capacity of the calorimeter b) Mass of sample c) Ti d) The molarity of the HCl e) Tfarrow_forwardPredict products for the Following organic rxn/s by writing the structurels of the correct products. Write above the line provided" your answer D2 ①CH3(CH2) 5 CH3 + D₂ (adequate)" + 2 mited) 19 Spark Spark por every item. 4 CH 3 11 3 CH 3 (CH2) 4 C-H + CH3OH CH2 CH3 + CH3 CH2OH 0 CH3 fou + KMnDy→ C43 + 2 KMn Dy→→ C-OH ") 0 C-OH 1110 (4.) 9+3 =C CH3 + HNO 3 0 + Heat> + CH3 C-OH + Heat CH2CH3 - 3 2 + D Heat H 3 CH 3 CH₂ CH₂ C = CH + 2 H₂ → 2 2arrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forward
- Q6: Using acetic acid as the acid, write the balanced chemical equation for the protonation of the two bases shown (on the -NH2). Include curved arrows to show the mechanism. O₂N- O₂N. -NH2 -NH2 a) Which of the two Bronsted bases above is the stronger base? Why? b) Identify the conjugate acids and conjugate bases for the reactants. c) Identify the Lewis acids and bases in the reactions.arrow_forwardQ5: For the two reactions below: a) Use curved electron-pushing arrows to show the mechanism for the reaction in the forward direction. Redraw the compounds to explicitly illustrate all bonds that are broken and all bonds that are formed. b) Label Bronsted acids and bases in the left side of the reactions. c) For reaction A, which anionic species is the weakest base? Which neutral compound is the stronger acid? Is the forward or reverse reaction favored? d) Label Lewis acids and bases, nucleophiles and electrophiles in the left side of the reactions. A. 용 CH3OH я хон CH3O OH B. HBr CH3ONa NaBr CH3OHarrow_forwardpotential energy Br b) Translate the Newman projection below to its wedge-and-dash drawing. F H. OH CH3 CI c) Isopentane (2-methylbutane) is a compound containing a branched carbon chain. Draw a Newman projection of six conformations about the C2-C3 bond of isopentane. On the curve of potential energy versus angle of internal rotation for isopentane, label each energy maximum and minimum with one of the conformations. 0° 。 F A B D C angle of internal rotation E F 360° (=0°) JDownlarrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





