
Concept explainers
The equilibrium constant for the conjugate acid-base pair
(a) calculate the absorbance at 430 nmand 600 nm for the following indicator concentrations:
3.00 × 10-4M,2.00 × 10-4M, 1.00 × 10-4M, 0.500 × 10-4 M, and 0.250 × 10-4M.
(b) plot absorbance as a function of indicator concentration.

(a)
Interpretation:
Absorbance values of the conjugate acid-base pair solutions of different concentrations at
Concept introduction:
The absorbance can be calculated using the following formula:
For the given conjugate acid-base pair, equilibrium can be written as,
Answer to Problem 13.11QAP
Explanation of Solution
Let’s use the equilibrium equation to find the concentrations of HIn and In-(denoted as [HIn] and [In-]) at a situation in which the total concentration (cHIn) is
Now, the absorbance values of this solution at 430 and 600 nm can be calculated as follows,
The cell length (b) is not given in the question, therefore taken as 1.00cm.
Similarly, other absorbance values can be calculated for solutions with different concentrations.
(b)
Interpretation:
Absorbance as a function of indicator concentration should be plotted.
Explanation of Solution
The data obtained is as follows:
From the data, the graph between absorbance and concentration can be plotted as follows:
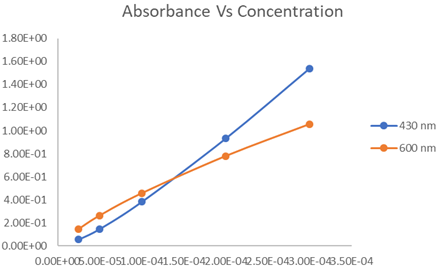
Here, blue curve indicates the plot at 430 nm and orange curve indicates the same at 600 nm.
Want to see more full solutions like this?
Chapter 13 Solutions
Principles of Instrumental Analysis
- Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 ----||| Molecule 4 Molecule 5 Molecule 6 none of the above mm..arrow_forwardUse the vapor-liquid equilibrium data at 1.0 atm. for methanol-water (Table 2-8 ) for the following: If the methanol vapor mole fraction is 0.600, what is the methanol liquid mole fraction? Is there an azeotrope in the methanol-water system at a pressure of 1.0 atmospheres? If water liquid mole fraction is 0.350, what is the water vapor mole fraction? What are the K values of methanol and of water at a methanol mole fraction in the liquid of 0.200? What is the relative volatility αM-W at a methanol mole fraction in the liquid of 0.200?arrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. || |II***** Molecule 1 | Molecule 4 none of the above Molecule 2 Molecule 3 Х mm... C ---||| *** Molecule 5 Molecule 6arrow_forward
- is SiBr4 Silicon (IV) tetra Bromine? is KClO2 potassium dihypochlorite ?arrow_forward"יוון HO" Br CI Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 Molecule 3 Br Br Br HO OH H CI OH ✓ Molecule 4 Molecule 5 Molecule 6 CI Br יייון H Br OH OH CI Br ☐ none of the above × Garrow_forwardUS2 Would this be Uranium (II) diSulfide?arrow_forward
- nomenclature for PU(SO4)3arrow_forwardLi2CrO4 is this Lithium (II) Chromatearrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. NH ** Molecule 1 NH Molecule 4 none of the above Х Molecule 3 Molecule 2 H N wwwwww.. HN Molecule 5 Molecule 6 HN R mw... N H ☐arrow_forward
- Nomenclature P4S3 Would this be tetraphsophorus tri sulfide?arrow_forwardDon't used Ai solutionarrow_forwardBenzene-toluene equilibrium is often approximated as αBT = 2.34. Generate the y-x diagram for this relative volatility. Also, generate the equilibrium data using Raoult’s law, and compare your results to these.arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


