
Concept explainers
13.106 Hydrazine,
(a) Which reaction occurs at the anode and which at the cathode?
(b) What is the net cell reaction?
(C) If the cell is to produce 0.50 A of current for 50.0 h, what mass in grams of hydrazine must be present?
(d) What mass in grams of
(b)
Interpretation:
To identify the net reaction.
Concept introduction:
- The net reaction is the total reaction including all reactants and products.
- It is obtained via addition of the half reactions.
Answer to Problem 13.106PAE
Solution:
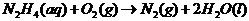
Explanation of Solution
In order to find out the reaction, we must include the reduction-oxidation reactions. They always take place together, since one donates electrons and the other receives them.
In this case, the oxidation reaction: hydrazine oxidation to nitrogen gas. The reduction is oxygen forming hydroxide ion via the addition of electron
Adding both reactions, getting rid of repeating/same units on the left/right side of the equation:
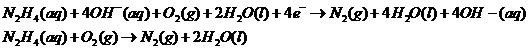
Therefore, the reaction taking place is:
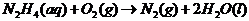
(c)
Interpretation:
To calculate the mass of hydrazine present in given amount of time and current.
Concept introduction:
- A stands for ampere, the amount of current.
- Time and current can be used to calculate total charge.
- The total charge relates mol of e- and Faraday constant.
Answer to Problem 13.106PAE
Solution:
Explanation of Solution
This consist of the following steps:
1st Step: Calculate the total amount of charge.
2nd Step: Calculate the total amount of moles of hydrazine
3rd Step: Calculate the mass of hydrazine
(d)
Interpretation:
To calculate the mass oxygen required
Concept introduction:
- Use stoichiometric ratios.
Answer to Problem 13.106PAE
Solution:
Explanation of Solution
Relate the moles of oxygen and hydrazine:
1st Step: Calculate total moles of oxygen required
The ratio is 1:1 since 1 mol of hydrazine requires 1 mol of Oxygen gas to react.
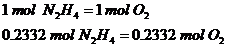
2nd Step: Calculate the total mass of oxygen gas
The mass is exactly the same as hydrazine since they have 1:1 ratio and similar molar masses.
Want to see more full solutions like this?
Chapter 13 Solutions
Bundle: Chemistry for Engineering Students, Loose-Leaf Version, 4th + OWLv2 with MindTap Reader with Student Solutions Manual, 1 term (6 months) Printed Access Card
- IV. Show the detailed synthesis strategy for the following compounds. a. CH3CH2CH2CH2Br CH3CH2CCH2CH2CH3arrow_forwardDo the electrons on the OH participate in resonance with the ring through a p orbital? How many pi electrons are in the ring, 4 (from the two double bonds) or 6 (including the electrons on the O)?arrow_forwardPredict and draw the product of the following organic reaction:arrow_forward
- Nonearrow_forwardRedraw the molecule below as a skeletal ("line") structure. Be sure to use wedge and dash bonds if necessary to accurately represent the direction of the bonds to ring substituents. Cl. Br Click and drag to start drawing a structure. : ☐ ☑ Parrow_forwardK m Choose the best reagents to complete the following reaction. L ZI 0 Problem 4 of 11 A 1. NaOH 2. CH3CH2CH2NH2 1. HCI B OH 2. CH3CH2CH2NH2 DII F1 F2 F3 F4 F5 A F6 C CH3CH2CH2NH2 1. SOCl2 D 2. CH3CH2CH2NH2 1. CH3CH2CH2NH2 E 2. SOCl2 Done PrtScn Home End FA FQ 510 * PgUp M Submit PgDn F11arrow_forward
- given cler asnwerarrow_forwardAdd curved arrows to the reactants in this reaction. A double-barbed curved arrow is used to represent the movement of a pair of electrons. Draw curved arrows. : 0: si H : OH :: H―0: Harrow_forwardConsider this step in a radical reaction: Br N O hv What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. O primary Otermination O initialization O electrophilic O none of the above × ☑arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





