
Concept explainers
13.106 Hydrazine,
(a) Which reaction occurs at the anode and which at the cathode?
(b) What is the net cell reaction?
(C) If the cell is to produce 0.50 A of current for 50.0 h, what mass in grams of hydrazine must be present?
(d) What mass in grams of
(b)
Interpretation:
To identify the net reaction.
Concept introduction:
- The net reaction is the total reaction including all reactants and products.
- It is obtained via addition of the half reactions.
Answer to Problem 13.104PAE
Solution:
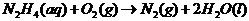
Explanation of Solution
In order to find out the reaction, we must include the reduction-oxidation reactions. They always take place together, since one donates electrons and the other receives them.
In this case, the oxidation reaction: hydrazine oxidation to nitrogen gas. The reduction is oxygen forming hydroxide ion via the addition of electron
Adding both reactions, getting rid of repeating/same units on the left/right side of the equation:
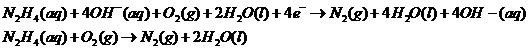
Therefore, the reaction taking place is:
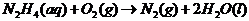
(c)
Interpretation:
To calculate the mass of hydrazine present in given amount of time and current.
Concept introduction:
- A stands for ampere, the amount of current.
- Time and current can be used to calculate total charge.
- The total charge relates mol of e- and Faraday constant.
Answer to Problem 13.104PAE
Solution:
Explanation of Solution
This consist of the following steps:
1st Step: Calculate the total amount of charge.
2nd Step: Calculate the total amount of moles of hydrazine
3rd Step: Calculate the mass of hydrazine
(d)
Interpretation:
To calculate the mass oxygen required
Concept introduction:
- Use stoichiometric ratios.
Answer to Problem 13.104PAE
Solution:
Explanation of Solution
Relate the moles of oxygen and hydrazine:
1st Step: Calculate total moles of oxygen required
The ratio is 1:1 since 1 mol of hydrazine requires 1 mol of Oxygen gas to react.
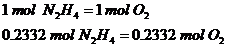
2nd Step: Calculate the total mass of oxygen gas
The mass is exactly the same as hydrazine since they have 1:1 ratio and similar molar masses.
Want to see more full solutions like this?
Chapter 13 Solutions
CHEM FOR ENGNRNG SDNTS (EBOOK) W/ACCES
- 2. Identify the reagents you would need to achieve the following. You may need to consider using a protecting group. HO 1. 2. 3. 4. 5. OH Br HOarrow_forwardBeF2 exists as a linear molecule. Which kind of hybrid orbitals does Be use in this compound? Use Orbital Diagrams to show how the orbitals are formed. (6)arrow_forwardPlease answer the questions and provide detailed explanations as well as a drawing to show the signals in the molecule.arrow_forward
- Propose an efficient synthesis for the following transformation: EN The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. t-BuOK B. Na2Cr2O7, H2SO4, H2O C. NBS, heat F. NaCN D. MeOH E. NaOH G. MeONa H. H2O I. 1) O3; 2) DMSarrow_forwardStereochemistry Identifying the enantiomer of a simple organic molecule 1/5 Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of t above box under the table. Br ま HO H 0 Molecule 1 Molecule 2 Molecule 3 OH H Br H H" Br OH Br Molecule 4 Br H OH + + OH Molecule 5 Br H OH none of the above Molecule 6 Br H... OHarrow_forwardPlease answer the questions and provide detailed explanations.arrow_forward
- Question 16 0/1 pts Choose the correct option for the following cycloaddition reaction. C CF3 CF3 CF3 CF3 The reaction is suprafacial/surafacial and forbidden The reaction is antarafacial/antarafacial and forbidden The reaction is antarafacial/antarafacial and allowed The reaction is suprafacial/surafacial and allowedarrow_forward1. Give the structures of the products obtained when the following are heated. Include stereochemistry where relevant. A NO2 + NO2 B + C N=C CEN + { 2. Which compounds would you heat together in order to synthesize the following?arrow_forwardExplain how myo-inositol is different from D-chiro-inositol. use scholarly sources and please hyperlink.arrow_forward
- What is the molarisuty of a 0.396 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 /mol.arrow_forwardProvide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly. Br ......Im OHarrow_forwardCan you please help me solve this problems. The top one is just drawing out the skeletal correct and then the bottom one is just very confusing to me and its quite small in the images. Can you enlarge it and explain it to me please. Thank You much (ME EX1) Prblm #33arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





