
Concept explainers
The mass spectrum of the following compound shows fragments at

Interpretation: The structures of the ions of a given compound that shows fragments at
Concept introduction: Molecular ion is the radical cation which is formed by ejection of electrons from a molecule when a beam of high-energy electrons bombarded on a molecule. Mass of the molecule is equal to
Answer to Problem 15P
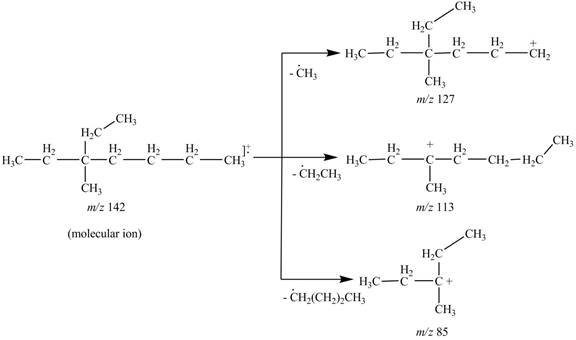
The fragments of 3-ethyl, 3-methylheptane at
Explanation of Solution
The ball and stick model of the given compound is,

Figure 1
Black colored atoms have four bonds. So, these are the carbon atoms. The grey colored balls have one bond. So, these are the hydrogen atoms. The molecular structure is,
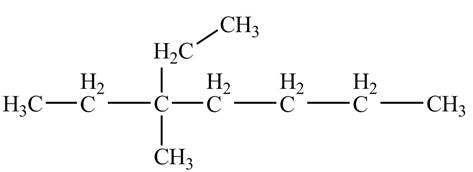
Figure 2
Hence, the ball and stick model of the given compound is 3-ethyl, 3-methylheptane. The mass of 3-ethyl, 3-methylheptane is follow.
Thus, the molecular ion peak of 3-ethyl, 3-methylheptane is observed at
The molecular ion
The molecular ion
The molecular ion
Hence, the fragments of 3-ethyl, 3-methylheptane at
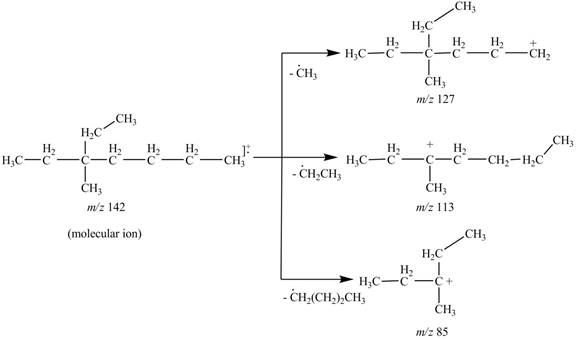
Figure 3
The fragments of 3-ethyl, 3-methylheptane at
Want to see more full solutions like this?
Chapter 12A Solutions
ORGANIC CHEMISTRY-STUDY GDE./SOL.MAN.
Additional Science Textbook Solutions
General, Organic, and Biological Chemistry - 4th edition
Anatomy & Physiology (6th Edition)
Biology: Life on Earth (11th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Organic Chemistry (8th Edition)
- Problem 5-48 Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C). OH H OH HO CH2OH Ascorbic acid O H Problem 5-49 Assign R or S stereochemistry to the chirality centers in the following Newman projections: H Cl H CH3 H3C. OH H3C (a) H H H3C (b) CH3 H Problem 5-52 Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each: OH OH (a) CH3CHCH2CH2CHCH3 CH3 H3C. -OH (c) H3C CH3 (b) Problem 5-66 Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States. H2N H IHH S Cephalexin N. CH3 CO₂Harrow_forwardSteps and explanationn please.arrow_forwardSteps and explanationn please.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

