
Concept explainers
Methane is to be adiabatically and reversibly compressed from 50 psia and 100°F to 500 psia. Calculate the specific work required for this compression treating the methane as an ideal gas with variable specific heats and using the departure charts.
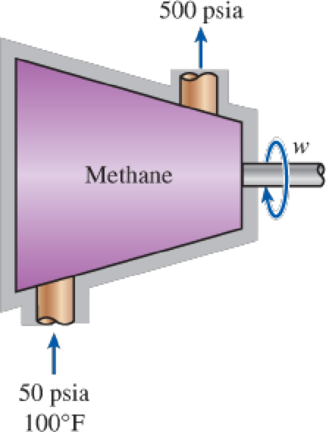
FIGURE P12–96E
The work input of the compressor per unit mass by treating the methane as an ideal gas with variable specific heats and using departure charts.
Answer to Problem 96RP
The work input of the compressor per unit mass by treating the methane as an ideal gas with variable specific heats is
The work input of the compressor per unit mass of the methane using departure charts is
Explanation of Solution
Refer the table A-2E (c), “Ideal gas specific heats of various common gases”.
The general empirical correlation is
Write the formula for enthalpy change in molar basis at ideal gas state
Here, specific heat capacity at constant pressure is
Write the formula for work input to the compressor
Here, molar mass of methane is
Write the formula for entropy change in molar basis at ideal gas state
Here, universal gas constant is
Calculate the reduced temperature
Here, critical temperature is
Calculate the reduced pressure
Here, critical pressure is
Calculate the reduced temperature
Here, critical temperature is
Calculate the reduced pressure
Here, critical pressure is
Write the formula for change in enthalpy
Here, change in enthalpy of ideal gas is
Refer Table A-1E, “Molar mass, gas constant, and critical-point properties”.
The critical temperature and critical pressure of the methane is as follows.
Refer table A-1E, “Molar mass, gas constant and critical properties table”.
The molar mass
Refer the table A-2E (c), “Ideal gas specific heats of various common gases”.
Obtain the empirical constants as follows.
Refer Table A-2E (a), “Ideal-gas specific heats of various common gases”.
The gas constant
The specific heat at constant pressure
The universal gas constant
Conclusion:
Convert the inlet temperature from degree Fahrenheit to Rankine.
It is given that the compression process in reversible adiabatic process. Hence, the change in entropy during the process is zero.
Substitute
By using Engineering Equation Solver (EES) or online calculator to solve the Equation (XI) and obtain the value of
Substitute
Substitute
Thus, the work input of the compressor per unit mass by treating the methane as an ideal gas with variable specific heats is
Substitute
Substitute
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Consider the final temperature
Substitute
Substitute
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Substitute
Here, the work input of the compressor is equal to the enthalpy difference.
Thus, the work input of the compressor per unit mass of the methane using departure charts is
Want to see more full solutions like this?
Chapter 12 Solutions
THERMODYNAMICS: ENG APPROACH LOOSELEAF
- 50 grams of water at 20ºC is converted into steam at 250ºC at constant atmospheric pressure. Assuming the heat capacity per gram of liquid water to remain constant at 4.2 J/g K and the heat of vaporization at 100ºC to be 226 J/g, and using Cp/R=a+bT+cT2, calculate the entropy change of the system. a=3.652, b=1.157x10-3 K-1, c=0.142x10-6 K-2arrow_forward7 kilograms of steam is being heated to a 600K and 10 MPa superheated vapor state from the initial 400K saturated vapor state. What is the change in entropy of the steam? Use the ChE Handbook for data.arrow_forwardPlease state if any steam table values are usedarrow_forward
- If a table is used, please show what table is used and where the values are found. Thank you in advance!arrow_forward1kg of liquid subcooled water at 90°C is left to cool down at a LARGE room at 25°C until thermodynamic equilibrium is reached. Calculate the entropy generation during the process. (Include the units) Answer:arrow_forwardA 50kg copper block is thrown into an isolated tank holding 80 L of water at a temperature of 75 degrees Celsius. Calculate the eventual equilibrium temperature and the process's total entropy change.arrow_forward
- A monatomic ideal gas that is initially at a pressure of 1.51x10^5 Pa and with a volume of 8.30×10-2 m3 is compressed adiabatically to a volume of 4.30×10^-2 m3. what is the final pressure? how much work is done by the gas during the compression?arrow_forward1 kg of AIR at P = 100 kPa is contained in a RIGID TANK made of IRON of mass 0.5 kg. Initially, the temperature of both the AlR and the TANK are 25°C. [a) Now, the TANK (air and the iron) is heated by an electric heater of power 100 W for 30 min. Calculate the entropy generation Sgen during the heating process. (Heat loss to the surrounding is prevented during this process.) b) Next, the tank is left to cool down in a VERY BIG room at T = 25°C. Calculate the total entropy generated Sgen [kJ/K] (both in the system and in the surrounding) during the cooling of the tank.arrow_forwardConsider an adiabatic turbine which produces 1375 kJ/kg of work assuming the water entering the turbine is at 10 MPa and 700°C and the water exits the turbine at 75 kPa. What is the phase description of the water at the outlet? Hint: Using the given information what second outlet property can you calculate? What is the specific entropy of the water at the inlet in kJ/kg K? What is the specific entropy of the water at the outlet, in kJ/kg K. Report your answer to two decimal places using rounding. How much specific entropy is generated in this process in kJ/kg K. Report your answer to three decimal places using rounding.arrow_forward
- Air at 3.2 MPa and 500°C is expanded in an adiabatic gas turbine to 0.2 MPa. Calculate the maximum work that this turbine can produce in kJ/kg. Use the table containing the ideal gas specific heats of various common gases. The maximum work that this turbine can produce is kJ/kg.arrow_forward0.03 m3 of nitrogen contained in a cylinder behind apiston is initially at 1.05 bar and 15ºC. The gas iscompressed isothermally and reversibly until the pressureis 4.2 bar. Calculate the change of entropy, the heat flow,and the work done, and sketch the process on a p-v andT-s diagrams. Assume nitrogen to act as a perfect gas.Molecular weight of nitrogen = 28.arrow_forwardDetermine the entropy for these states. R-134a, T = 25°C, v = 0.01 m3/kgarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





