
Interpretation:
The reagent that has to be used to achieve the given transformation has to be identified.
Concept Introduction:

Hydration of Alkynes:
Alkynes undergo hydration reaction in presence of acid and mercuric sulfate as catalyst to form an enol as the initial product. The formed enol gets converted fast into keto due to keto-enol tautomerism.
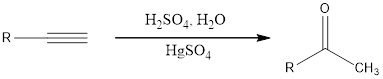
Hydration of alkynes through the method said above is a Markovnikov addition.
Hydroboration-oxidation of Alkynes:
Alkynes undergo hydroboration-oxidation reaction in presence of dialkyl borane, hydrogen peroxide and sodium hydroxide to form an enol as the initial product. The formed enol gets converted fast into keto due to keto-enol tautomerism. Ketone and enol are constitutional isomers. If the alkyne under consideration is a terminal alkyne, then
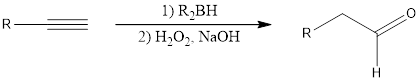
Hydroboration-oxidation of alkynes through the method said above is an anti-Markovnikov addition.
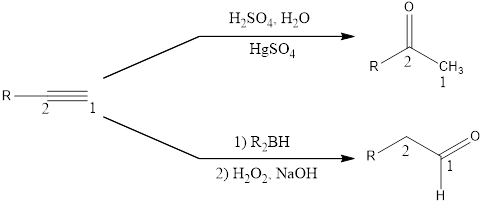
Acid catalyzed hydration of alkynes installs the carbonyl group at the C2 position, while hydroboration-oxidation installs the carbonyl group in C1 position.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Organic Chemistry As a Second Language: First Semester Topics
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





