
EBK CHEMISTRY
12th Edition
ISBN: 9780133911312
Author: Timberlake
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Question
Chapter 12.4, Problem 12.28QAP
Interpretation Introduction
(a)
To determine: The condensed structural formula of compound for the 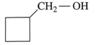 is oxidised.
is oxidised.
Interpretation Introduction
(b)
To determine: The condensed structural formula of compound for the aldehydes and caboxylic acid formed when the given compound- is oxidised.
Interpretation Introduction
(c)
To determine: The condensed structural formula of compound for the aldehydes and
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show work..don't give Ai generated solution...
Label the spectrum with spectroscopy
Please correct answer and don't use hand rating and don't use Ai solution
Chapter 12 Solutions
EBK CHEMISTRY
Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Prob. 12.3QAPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Prob. 12.5QAPCh. 12.1 - Prob. 12.6QAPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Draw the condensed structural formula, or...Ch. 12.2 - Prob. 12.9QAPCh. 12.2 - Prob. 12.10QAP
Ch. 12.2 - Prob. 12.11QAPCh. 12.2 - Prob. 12.12QAPCh. 12.2 - Prob. 12.13QAPCh. 12.2 - Give an explanation for each of the following...Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Prob. 12.17QAPCh. 12.3 - Prob. 12.18QAPCh. 12.3 - Draw the condensed structural formula for each of...Ch. 12.3 - Prob. 12.20QAPCh. 12.3 - Which compound in each of the following pairs...Ch. 12.3 - Which compound in each of the following pairs...Ch. 12.4 - Prob. 12.23QAPCh. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Prob. 12.25QAPCh. 12.4 - Prob. 12.26QAPCh. 12.4 - Draw the condensed structural formulas for the...Ch. 12.4 - Prob. 12.28QAPCh. 12.4 - Prob. 12.29QAPCh. 12.4 - Prob. 12.30QAPCh. 12.4 - Write the balanced chemical equation for the...Ch. 12.4 - Write the balanced chemical equation for the...Ch. 12 - Prob. 12.33UTCCh. 12 - The compound frambinone has the taste of...Ch. 12 - Prob. 12.35UTCCh. 12 - Prob. 12.36UTCCh. 12 - Prob. 12.37UTCCh. 12 - Prob. 12.38UTCCh. 12 - Prob. 12.39UTCCh. 12 - Prob. 12.40UTCCh. 12 - Prob. 12.41AQAPCh. 12 - Prob. 12.42AQAPCh. 12 - Prob. 12.43AQAPCh. 12 - Prob. 12.44AQAPCh. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Prob. 12.47AQAPCh. 12 - Which compound in each pair would be more soluble...Ch. 12 - Prob. 12.49AQAPCh. 12 - Prob. 12.50AQAPCh. 12 - Prob. 12.51AQAPCh. 12 - Draw the condensed structural or line-angle...Ch. 12 - Prob. 12.53AQAPCh. 12 - Prob. 12.54AQAPCh. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Prob. 12.59AQAPCh. 12 - Prob. 12.60AQAPCh. 12 - Prob. 12.61CQCh. 12 - Draw the condensed structural formulas and give...Ch. 12 - A compound with the formula C4H8O is synthesized...Ch. 12 - A compound with the formula C5H10O oxidizes to...Ch. 12 - Compound A is a primary alcohol whose formula is...Ch. 12 - Prob. 12.66CQCh. 12 - Prob. 21CICh. 12 - Prob. 22CICh. 12 - Prob. 23CICh. 12 - Prob. 24CICh. 12 - Prob. 25CICh. 12 - lonone is a compound that gives violets their...
Knowledge Booster
Similar questions
- Label the spectrum with spectroscopyarrow_forwardLabel the spectrum with spectroscopyarrow_forwardQ1: Draw the most stable and the least stable Newman projections about the C2-C3 bond for each of the following isomers (A-C). Are the barriers to rotation identical for enantiomers A and B? How about the diastereomers (A versus C or B versus C)? enantiomers H Br H Br (S) CH3 H3C (S) (R) CH3 H3C H Br A Br H C H Br H3C (R) B (R)CH3 H Br H Br H3C (R) (S) CH3 Br H D identicalarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY