
EBK CHEMISTRY
12th Edition
ISBN: 9780133911312
Author: Timberlake
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Question
Chapter 12.2, Problem 12.9QAP
Interpretation Introduction
(a)
To determine: If the alcohol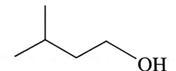 is a primary (), secondary () or tertiary () alcohol.
is a primary (), secondary () or tertiary () alcohol.
Interpretation Introduction
(b)
To determine: If the alcohol is a primary (), secondary () or tertiary () alcohol.
Interpretation Introduction
(c)
To determine: If the alcohol 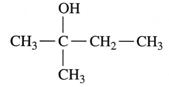 is a primary (), secondary () or tertiary () alcohol.
is a primary (), secondary () or tertiary () alcohol.
Interpretation Introduction
(d)
To determine: If the alcohol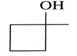 is a primary (), secondary () or tertiary () alcohol.
is a primary (), secondary () or tertiary () alcohol.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please help me calculate the undiluted samples ppm concentration.
My calculations were 280.11 ppm. Please see if I did my math correctly using the following standard curve.
Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EVSJL_W0qrxMkUjK2J3xMUEBHDu0UM1vPKQ-bc9HTcYXDQ?e=hVuPC4
Provide an IUPAC name for each of the compounds shown.
(Specify (E)/(Z) stereochemistry, if relevant, for straight chain alkenes only. Pay attention to
commas, dashes, etc.)
H₁₂C
C(CH3)3
C=C
H3C
CH3
CH3CH2CH
CI
CH3
Submit Answer
Retry Entire Group
2 more group attempts remaining
Previous
Next
Arrange the following compounds / ions in increasing nucleophilicity (least to
most nucleophilic)
CH3NH2
CH3C=C:
CH3COO
1
2
3
5
Multiple Choice 1 point
1, 2, 3
2, 1, 3
3, 1, 2
2, 3, 1
The other answers are not correct
0000
Chapter 12 Solutions
EBK CHEMISTRY
Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Prob. 12.3QAPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Prob. 12.5QAPCh. 12.1 - Prob. 12.6QAPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Draw the condensed structural formula, or...Ch. 12.2 - Prob. 12.9QAPCh. 12.2 - Prob. 12.10QAP
Ch. 12.2 - Prob. 12.11QAPCh. 12.2 - Prob. 12.12QAPCh. 12.2 - Prob. 12.13QAPCh. 12.2 - Give an explanation for each of the following...Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Prob. 12.17QAPCh. 12.3 - Prob. 12.18QAPCh. 12.3 - Draw the condensed structural formula for each of...Ch. 12.3 - Prob. 12.20QAPCh. 12.3 - Which compound in each of the following pairs...Ch. 12.3 - Which compound in each of the following pairs...Ch. 12.4 - Prob. 12.23QAPCh. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Prob. 12.25QAPCh. 12.4 - Prob. 12.26QAPCh. 12.4 - Draw the condensed structural formulas for the...Ch. 12.4 - Prob. 12.28QAPCh. 12.4 - Prob. 12.29QAPCh. 12.4 - Prob. 12.30QAPCh. 12.4 - Write the balanced chemical equation for the...Ch. 12.4 - Write the balanced chemical equation for the...Ch. 12 - Prob. 12.33UTCCh. 12 - The compound frambinone has the taste of...Ch. 12 - Prob. 12.35UTCCh. 12 - Prob. 12.36UTCCh. 12 - Prob. 12.37UTCCh. 12 - Prob. 12.38UTCCh. 12 - Prob. 12.39UTCCh. 12 - Prob. 12.40UTCCh. 12 - Prob. 12.41AQAPCh. 12 - Prob. 12.42AQAPCh. 12 - Prob. 12.43AQAPCh. 12 - Prob. 12.44AQAPCh. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Prob. 12.47AQAPCh. 12 - Which compound in each pair would be more soluble...Ch. 12 - Prob. 12.49AQAPCh. 12 - Prob. 12.50AQAPCh. 12 - Prob. 12.51AQAPCh. 12 - Draw the condensed structural or line-angle...Ch. 12 - Prob. 12.53AQAPCh. 12 - Prob. 12.54AQAPCh. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Prob. 12.59AQAPCh. 12 - Prob. 12.60AQAPCh. 12 - Prob. 12.61CQCh. 12 - Draw the condensed structural formulas and give...Ch. 12 - A compound with the formula C4H8O is synthesized...Ch. 12 - A compound with the formula C5H10O oxidizes to...Ch. 12 - Compound A is a primary alcohol whose formula is...Ch. 12 - Prob. 12.66CQCh. 12 - Prob. 21CICh. 12 - Prob. 22CICh. 12 - Prob. 23CICh. 12 - Prob. 24CICh. 12 - Prob. 25CICh. 12 - lonone is a compound that gives violets their...
Knowledge Booster
Similar questions
- curved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the cured electron-pushing arrows for thw following reaction or mechanistic steps. be sure to account for all bond-breaking and bond making stepsarrow_forwardUsing the graphs could you help me explain the answers. I assumed that both graphs are proportional to the inverse of time, I think. Could you please help me.arrow_forwardSynthesis of Dibenzalacetone [References] Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. Question 1 1 pt Question 2 1 pt Question 3 1 pt H Question 4 1 pt Question 5 1 pt Question 6 1 pt Question 7 1pt Question 8 1 pt Progress: 7/8 items Que Feb 24 at You do not have to consider stereochemistry. . Draw the enolate ion in its carbanion form. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ⚫ Separate multiple reactants using the + sign from the drop-down menu. ? 4arrow_forward
- Shown below is the mechanism presented for the formation of biasplatin in reference 1 from the Background and Experiment document. The amounts used of each reactant are shown. Either draw or describe a better alternative to this mechanism. (Note that the first step represents two steps combined and the proton loss is not even shown; fixing these is not the desired improvement.) (Hints: The first step is correct, the second step is not; and the amount of the anhydride is in large excess to serve a purpose.)arrow_forwardHi I need help on the question provided in the image.arrow_forwardDraw a reasonable mechanism for the following reaction:arrow_forward
- Draw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward
- 20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward20.00 mL of 0.150 M HCl is titrated with 37.75 mL of NaOH. What is the molarity of the NaOH?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY