
Concept explainers
The chapter sections to review are shown in parentheses at the end of each problem.
12.87 Match the diagrams with the following: (12.1)
a. a polar solute and a polar solvent
b. a nonpolar solute and a polar solvent
c. a nonpolar solute and a nonpolar solvent
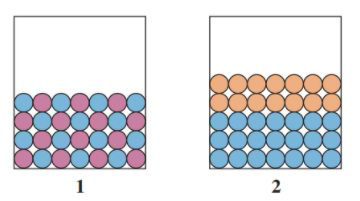 c
c
Interpretation:
The problem shows two diagrams with different type of solute-solvents.
Concept introduction:
- Like dissolves like is typically true between species
- Polar-polar solvents will always mix as well as Nonpolar-nonpolar solvents
- Nonpolar-Polar mixture will never mix, will form two phases.
To Match:The diagrams which shows the best option.
Answer to Problem 87UTC
Solution:
- 1
- 2
- 1
Explanation of Solution
There are different type of interaction between polar-polar, nonpolar-nonpolar and nonpolar-polar species. Therefore, one must ensure that the solute and solvent are well defined.
The solute is always in smaller amount than the solvent. Image (1) states an uniform mixture of species, whereas image (2) shows two phase, no mixture.
For any given mixture, there must be then either a polar-polar or a nonpolar-nonpolar solute/solvent system.
Image (2) shows the only combination possible, a nonpolar-polar mixture.
Always ensure to identify the solute, solvent and the type of interaction between them.
Want to see more full solutions like this?
Chapter 12 Solutions
Basic Chemistry
- The following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forwardPredict the product of the following reactions: O 0= excess Х Кон ОН H+ H+ Iarrow_forward
- How many chiral centers/stereocenters are there in the following molecule? 1 2 3 4arrow_forwardWhich of these correspond to the molecule: 2,5-dimethylheptanearrow_forwardGiven the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





