
(a)
Interpretation:
Gas with greater density in pairs below has to be determined.
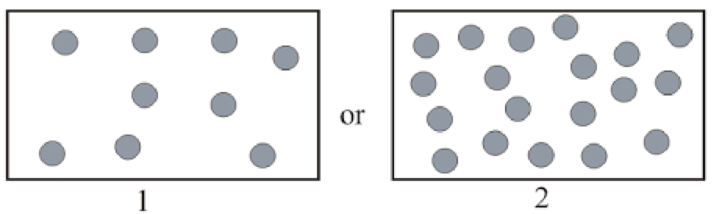
Concept Introduction:
Density of gas represents mass of gas present in unit volume. Expression of density is as follows:
Here,
Here,
Modified ideal gas equation for expression of density is as follows:
Here,
(b)
Interpretation:
Gas with greater density in pairs below has to be determined.
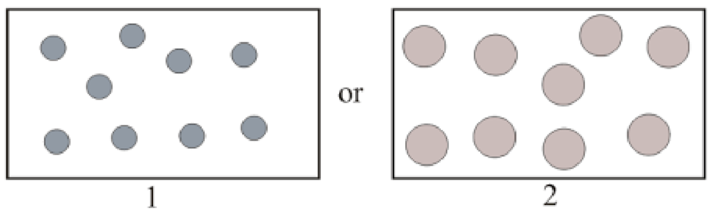
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- How would the graph in Figure 9.12 change if the number of moles of gas in the sample used to determine the curve were doubled?arrow_forwardf a gaseous mixture is made of 3.50gof He and 5.75gof Ar in an evacuated 2.05Lcontainer at 25C, what will be the partial pressure of Ar in the container?arrow_forwardFor which of the following gases should the correction for the molecular volume be largest: CO,CO2,H2,He,NH3,SF6?arrow_forward
- Which of the following gas samples would have the largest volume, if all samples are at the same temperature and pressure? a 263g of Xe; b 51023 molecules of H2; c 4.00moles of CO2; d they would all have the same volume.arrow_forward107 A soft drink can’s label indicates that the volume of the soda it contains is 12 oz or 355 mL. There is probably some empty space at the top of the can. Describe what you can measure and how that measurement allows you to determine the actual density of the soda.arrow_forwardAnalyze When nitroglycerin (C3H5N3O9) explodes, itdecomposes into the following gases: CO2 , N2 , NO, and H2O . If 239 g of nitroglycerin explodes, what volumewill the mixture of gaseous products occupy at 1.00 atmpressure and 2678°C?arrow_forward
- f 5.l2gof oxygen gas occupies a volume of 6.21Lat a certain temperature and pressure, what volume will 25.0gof oxygen gas occupy under the same conditions?arrow_forward7. The following are true to gas molecules EXCEPT for A. Gases exert pressure. B. Gases fill their container uniformly. C. Gases mix with one another to form a homogeneous mixture. D. Gases can be compressed to a smaller volume and in turn decrease density. 8. Which gas physical property is TRUE? A. Gases assume the area of their containers. B. Gases are the least compressible of the states of matter. C. Gases will mix unevenly when confined to a container. D. Gases have much lower density than liquids and solids. 9. Ideal gas conforms to the pattern of gas behavior as defined by the following theory and law EXCEPT for A. Kinetic Molecular Theory B. Law of Thermodynamics 10. Real gases can only obey this law at very high T and very low P. A. Combined Gas Law C Murphy's Law D. Maxwell-Boltzmann Distribution C Law of Thermodynamics D. Murphy's Law B. Ideal Gas Lawarrow_forwardFor many purposes we can treat ammonia (NH3) as an ideal gas at temperatures above its boiling point of -33.C. Suppose the pressure on a 44 g sample of ammonia gas at 8°C is reduced to 1/3 its initial value. A. Is it possible to change the temperature of the ammonia at the same time such that the volume of the gas doesn’t change? Yes or no? B. If you answered yes, calculate the new temperature of the gas round your answer to the nearest degrees Celsiusarrow_forward
- The stopcock connecting a 3.51 L bulb containing carbon dioxide gas at a pressure of 9.35 atm, and a 5.88 L bulb containing krypton gas at a pressure of 4.71 atm, is opened and the gases are allowed to mix. Assuming that the temperature remains constant, the final pressure in the system is ____ atm.arrow_forwardGive a brief discussion and trivia for Combined Gas Law and Ideal Gas law. Give at least 30 sentences. Also give some examples for each gasses.arrow_forwardA 10.0 g sample of O2 at standard temperature and pressure (STP) would occupy what volume in liters?arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





