
Concept explainers
(a)
Interpretation:
The Lewis structure of molecules or ions, spatial orientation of electron pairs and the simple geometric structure indicating the approximate bond angles of that molecules or ions should be determined.
Concept Introduction:
Lewis structure represents the valence electron arrangement around the atoms of the molecule. Lewis structure can be drawn for ionic or covalent bonds.
When predicting the geometric structure, VSPER theory is used. The three-dimensional structure of atoms in.
Answer to Problem 46CR
Explanation of Solution
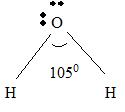
For H2 O
Part A Step 1 →
Sum(total atoms)
Step 2 →
Pair of electrons =
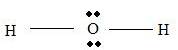
Electron pairs for bonds =
Step 3 → Remaining electron pairs = 2
Part B Step 1 → Lewis structure
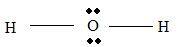
Step 2 → 4 electron pairs around
Step 3 → But around the
No: of bonding pairs =
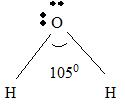
No: of non- bonding pairs =
Step 4 → Name of the structure = V-shaped or Bend.
(b)
Interpretation:
The Lewis structure of molecules or ions, spatial orientation of electron pairs and the simple geometric structure indicating the approximate bond angles of that molecules or ions should be determined.
Concept Introduction:
Lewis structure represents the valence electron arrangement around the atoms of the molecule. Lewis structure can be drawn for ionic or covalent bonds.
When predicting the geometric structure, VSPER theory is used. The three-dimensional structure of atoms in.
Answer to Problem 46CR
Explanation of Solution
For PH3 Part A Step 1 →
Sum
Step 2 → Pair of electrons =
Electron pairs for bonds =
Step 3 → Remain electron pairs =
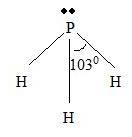
Part B Step 1 → Lewis structure
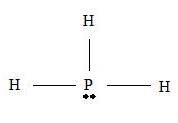
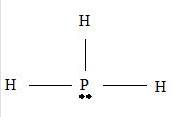
Step 2 →
Step 3 → But around the
No: of non- bonding pairs = 1
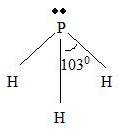
Step 4 → Name of the structure = Trigonal pyramid.
(c)
Interpretation:
The Lewis structure of molecules or ions, spatial orientation of electron pairs and the simple geometric structure indicating the approximate bond angles of that molecules or ions should be determined.
Concept Introduction:
Lewis structure represents the valence electron arrangement around the atoms of the molecule. Lewis structure can be drawn for ionic or covalent bonds.
When predicting the geometric structure, VSPER theory is used. The three-dimensional structure of atoms in.
Answer to Problem 46CR
Explanation of Solution
CBr4 Part A. Step 1 →
Sum = 32
Step 2 → Pair of electrons =
Electron pairs for bonds =
Step 3 → Remain electron pairs =
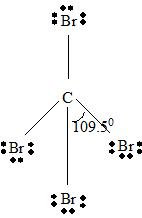
Part B Step 1 → Lewis structure
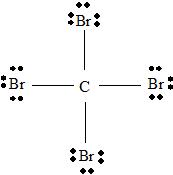
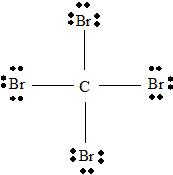
Step 2 →
Step 3 → But around the
No: of non- bonding pairs =
Step 4 → Name of the structure = Tetrahedral.
(d)
Interpretation:
The Lewis structure of molecules or ions, spatial orientation of electron pairs and the simple geometric structure indicating the approximate bond angles of that molecules or ions should be determined.
Concept Introduction:
Lewis structure represents the valence electron arrangement around the atoms of the molecule. Lewis structure can be drawn for ionic or covalent bonds.
When predicting the geometric structure, VSPER theory is used. The three-dimensional structure of atoms in.
Answer to Problem 46CR
Explanation of Solution
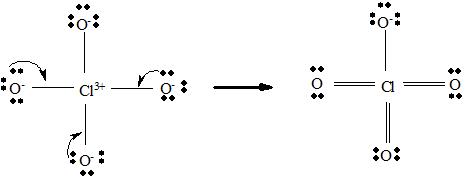
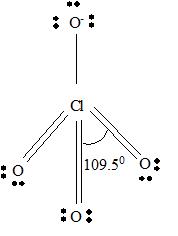
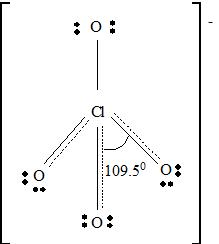
For ClO4 -
Part A. Step 1 →
Sum =
Step 2 → Pair of electrons =
Electron pairs for bonds =
Step 3 → Remain electron pairs =
Part B Step 1 → Lewis structure
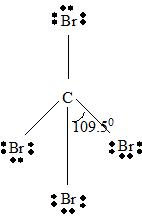
Step 2 →
Step 3 → But around the
No: of non- bonding pairs =
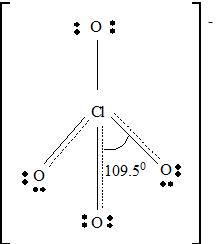
Step 4 → Name of the structure = Tetrahedral
But considering resonance, the exact position of three p bonds cannot be determined exactly. It provides a p cloud over the molecule. So that a resonance hybrid determines the exact structure of ClO4 - molecule.
(e)
Interpretation:
The Lewis structure of molecules or ions, spatial orientation of electron pairs and the simple geometric structure indicating the approximate bond angles of that molecules or ions should be determined.
Concept Introduction:
Lewis structure represents the valence electron arrangement around the atoms of the molecule. Lewis structure can be drawn for ionic or covalent bonds.
When predicting the geometric structure, VSPER theory is used. The three-dimensional structure of atoms in.
Answer to Problem 46CR
Explanation of Solution
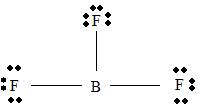
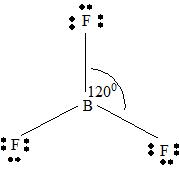
BF3
Part A. Step 1 →
Sum =
Step 2 → Pair of electrons =
Electron pairs for bonds =
Step 3 → Remain electron pairs =
Part B Step 1 → Lewis structure
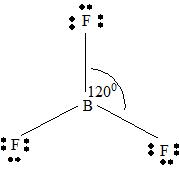
Step 2 →
Step 3 → Also around the
No: of bonding pairs =
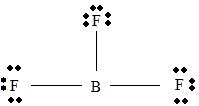
No: of non- bonding pairs =
Step 4 → Name of the structure = Trigonal planar.
(f)
Interpretation:
The Lewis structure of molecules or ions, spatial orientation of electron pairs and the simple geometric structure indicating the approximate bond angles of that molecules or ions should be determined.
Concept Introduction:
Lewis structure represents the valence electron arrangement around the atoms of the molecule. Lewis structure can be drawn for ionic or covalent bonds.
When predicting the geometric structure, VSPER theory is used. The three-dimensional structure of atoms in.
Answer to Problem 46CR
Explanation of Solution
BeF2
Part A. Step 1 →
Sum =
Step 2 → Pair of electrons =
Electron pairs for bonds =
Step 3 → Remain electron pairs =
Part B Step 1 → Lewis structure
Step 2 →
Step 3 → Also around the
No: of non- bonding pairs =
Step 4 → Name of the structure = Linear
 .
.
Want to see more full solutions like this?
Chapter 12 Solutions
Introductory Chemistry: Foundation - Text (Looseleaf)
- Q5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forwardCalculate the proton and carbon chemical shifts for this structurearrow_forwardA. B. b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion and B. is a neutral molecule. One of these molecules is a highly reactive compound first characterized in frozen noble gas matrices, that self-reacts rapidly at temperatures above liquid nitrogen temperature. The other compound was isolated at room temperature in the early 1960s, and is a stable ligand used in organometallic chemistry. Which molecule is the more stable molecule, and why?arrow_forward
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





