
Concept explainers
(a)
Interpretation:
Determine the electron configuration of atoms using the appropriate noble gas configuration of the core electrons.
Concept Introduction:
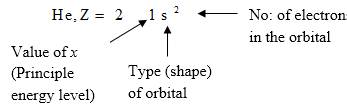
Electron configuration describes the positions of electrons of an atom. These positions were explained by several models developed by different scientists. Principal energy level, Type (shape) of orbital and No: of electrons in the orbital are respectively represented by an atom’s electron configuration.
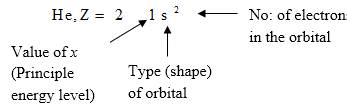
Answer to Problem 44CR
Using noble gas configuration(
Explanation of Solution
Order in which orbitals fill to produce the atoms in periodic table as follows:
There are only one
Noble gas configurations are as follows:
Period No
Thus, electronic configuration of Sr will be:
Using noble gas configuration(
(b)
Interpretation:
Determine the electron configuration of atoms using the appropriate noble gas configuration of the core electrons.
Concept Introduction:
Electron configuration describes the positions of electrons of an atom. These positions were explained by several models developed by different scientists. Principal energy level, Type (shape) of orbital and No: of electrons in the orbital are respectively represented by an atom’s electron configuration.
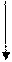
Answer to Problem 44CR
Using noble gas configuration(
Explanation of Solution
Order in which orbitals fill to produce the atoms in periodic table as follows:
There are only one
Noble gas configurations are as follows:
Period No
Thus, the electronic configuration of Al will be:
Using noble gas configuration(
(c)
Interpretation:
Determine the electron configuration of atoms using the appropriate noble gas configuration of the core electrons.
Concept Introduction:
Electron configuration describes the positions of electrons of an atom. These positions were explained by several models developed by different scientists. Principal energy level, Type (shape) of orbital and No: of electrons in the orbital are respectively represented by an atom’s electron configuration.
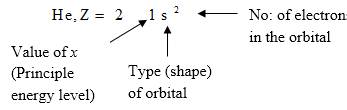
Answer to Problem 44CR
Using noble gas configuration(
Explanation of Solution
Order in which orbitals fill to produce the atoms in periodic table as follows:
There are only one
Noble gas configurations are as follows:
Period No
Thus, the electronic configuration of Cl will be:
Using noble gas configuration(
(d)
Interpretation:
Determine the electron configuration of atoms using the appropriate noble gas configuration of the core electrons.
Concept Introduction:
Electron configuration describes the positions of electrons of an atom. These positions were explained by several models developed by different scientists. Principal energy level, Type (shape) of orbital and No: of electrons in the orbital are respectively represented by an atom’s electron configuration.
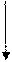
Answer to Problem 44CR
Using noble gas configuration(
Explanation of Solution
Order in which orbitals fill to produce the atoms in periodic table as follows:
There are only one
Noble gas configurations are as follows:
Period No
Thus, the electronic configuration of K will be:
Using noble gas configuration(
(e)
Interpretation:
Determine the electron configuration of atoms using the appropriate noble gas configuration of the core electrons.
Concept Introduction:
Electron configuration describes the positions of electrons of an atom. These positions were explained by several models developed by different scientists. Principal energy level, Type (shape) of orbital and No: of electrons in the orbital are respectively represented by an atom’s electron configuration.
Answer to Problem 44CR
Using noble gas configuration(
Explanation of Solution
Order in which orbitals fill to produce the atoms in periodic table as follows:
There are only one
Noble gas configurations are as follows:
Period No
Thus, the electronic configuration of S will be:
Using noble gas configuration(
(f)
Interpretation:
Determine the electron configuration of atoms using the appropriate noble gas configuration of the core electrons.
Concept Introduction:
Electron configuration describes the positions of electrons of an atom. These positions were explained by several models developed by different scientists. Principal energy level, Type (shape) of orbital and No: of electrons in the orbital are respectively represented by an atom’s electron configuration.
Answer to Problem 44CR
Using noble gas configuration(
Explanation of Solution
Order in which orbitals fill to produce the atoms in periodic table as follows:
There are only one
Noble gas configurations are as follows:
Period No
Thus, the electronic configuration of As will be:
Using noble gas configuration(
Want to see more full solutions like this?
Chapter 12 Solutions
Introductory Chemistry: Foundation - Text (Looseleaf)
- Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forwardDraw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forward
- 3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward6. Design the most efficient synthesis of the following product starting from phenot Provide the reaction conditions for each step (more than one step is required) and explain the selectivity of each reaction. NO MECHANISMS ARE REQUIRED. OH step(s) CIarrow_forward
- What is the skeletal structure of the product of the following organic reaction?arrow_forwardIf a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWhat is the major organic product of the following nucleophilic acyl substitution reaction of an acid chloride below?arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning  Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





