
ORG CHEM CONNECT CARD
6th Edition
ISBN: 9781264860746
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 45P
Draw the products formed in each oxidative cleavage.
a. 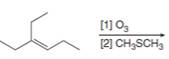 c.
c. 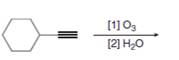
b. 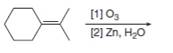 d.
d. 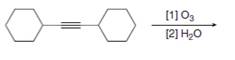
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Q7: Identify the functional groups in these molecules
a)
CH 3
b) Aspirin:
HO
'N'
Capsaicin
HO
O
CH3
CH 3
Q2: Name the following alkanes
1. Complete the following table in your laboratory notebook.
Substance
Formula
Methanol
CH3OH
Ethanol
C2H5OH
1-Propanol
C3H7OH
1-Butanol
C4H9OH
Pentane
C5H12
Hexane
C6H14
Water
H₂O
Acetone
C3H60
Structural Formula
Molecular Weight
(g/mol)
Hydrogen Bond
(Yes or No)
Chapter 12 Solutions
ORG CHEM CONNECT CARD
Ch. 12.1 - Prob. 1PCh. 12.3 - Problem 12.2 What alkane is formed when each...Ch. 12.3 - Prob. 3PCh. 12.3 - Prob. 4PCh. 12.3 - Prob. 5PCh. 12.3 - Prob. 6PCh. 12.3 - Compound Molecular formula before...Ch. 12.4 - Problem 12.8 Draw the products formed when...Ch. 12.5 - Prob. 9PCh. 12.5 - Prob. 10P
Ch. 12.5 - Problem 12.11 (a) Draw the structure of a compound...Ch. 12.5 - Prob. 12PCh. 12.5 - Prob. 13PCh. 12.6 - Problem 12.14 Draw the products of each...Ch. 12.8 - Prob. 15PCh. 12.8 - Problem 12.16 Draw all stereoisomers formed when...Ch. 12.9 - Prob. 17PCh. 12.9 - Problem 12.18 Draw the products formed when both...Ch. 12.10 - Problem 12.19 Draw the products formed when each...Ch. 12.10 - Prob. 20PCh. 12.10 - Prob. 21PCh. 12.11 - Problem 12.22 Draw the products formed when each...Ch. 12.11 - Prob. 23PCh. 12.12 - Problem 12.24 Draw the organic products in each of...Ch. 12.13 - Prob. 25PCh. 12 - 12.29 Draw the products formed when A is treated...Ch. 12 - Prob. 30PCh. 12 - Prob. 31PCh. 12 - Prob. 32PCh. 12 - Prob. 33PCh. 12 - Draw the organic products formed when cyclopentene...Ch. 12 - Draw the organic products formed when allylic...Ch. 12 - Prob. 39PCh. 12 - Prob. 40PCh. 12 - Prob. 41PCh. 12 - What alkene is needed to synthesize each 1,2-diol...Ch. 12 - 12.48 Draw the products formed in each oxidative...Ch. 12 - What alkene or alkyne yields each set of products...Ch. 12 - Prob. 47PCh. 12 - Prob. 48PCh. 12 - Prob. 49PCh. 12 - Prob. 50PCh. 12 - 12.57 Draw the product of each asymmetric...Ch. 12 - 12.60 Identify A in the following reaction...Ch. 12 - Prob. 58PCh. 12 - 12.62 It is sometimes necessary to isomerize a cis...Ch. 12 - 12.63 Devise a synthesis of each compound from...Ch. 12 - Prob. 61P
Additional Science Textbook Solutions
Find more solutions based on key concepts
An electric motor has an effective resistance of 32.0 and an inductive reactance of 45.0 when working under l...
Fundamentals of Physics Extended
Give the IUPAC name for each compound.
Organic Chemistry
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q1: Compare the relative acidity in each pair of compounds. Briefly explain. (a) CH3OH vs NH 3 (b) HF vs CH3COOH (c) NH3 vs CH4 (d) HCI vs HI (e) CH3COOH vs CH3SH (f) H₂C=CH2 vs CH3 CH3 (g) compare the acidity of the two bolded hydrogens O. H N- (h) compare the acidity of the two bolded hydrogens, draw resonance structures to explain H H Harrow_forwardQ3: Rank the following molecules in order of decreasing boiling point: (a) 3-methylheptane; (b) octane; (c) 2,4-dimethylhexane; (d) 2,2,4-trimethylpentane.arrow_forwardQ5: Conformations of Alkanes a) Draw a Newman Projection of the compound below about the C2-C3 bond. H3C Cli... H IIIH Br CH3arrow_forward
- The ability of atoms to associate with each other depends ona) the electronic structure and its spatial orientation.b) the electron affinity.c) The other two answers are correct.arrow_forwardWhat is the final volume after you reach the final temperature? I put 1.73 but the answer is wrong not sure why The initial volume of gas is 1.60 LL , the initial temperature of the gas is 23.0 °C°C , and the system is in equilibrium with an external pressure of 1.2 bar (given by the sum of a 1 bar atmospheric pressure and a 0.2 bar pressure due to a brick that rests on top of the piston). Then, as you did in Exercise 1, you heat the gas slowly until the temperature reaches 48.2 °Carrow_forwardQ4: Identify the type of Carbon ( methyl, primary, secondary, etc. ) indicated by this arrow.arrow_forward
- Q3: Curved Arrows, Lewis Acids & Bases, Nucleophiles and Electrophiles Considering the following reactions: a) Predict the products to complete the reactions. b) Use curved electron-pushing arrows to show the mechanism for the reaction in the forward direction. Redraw some of the compounds to explicitly illustrate all bonds that are broken and all bonds that are formed. c) Label Lewis acids and bases, nucleophiles and electrophiles in the reactions. A. S + AICI 3 B. + H₂Oarrow_forward3. A thermometer is placed in a test tube of chipped ice at -5.0 °C. The temperature is recorded at the time intervals shown below until room temperature is reached. Plot the data given below on graph paper and explain all flat, horizontal portions of the curve. Plot time on the X-axis! Time (min) Temperature (°C) 0 -5.0 2 -2.5 4 -1.0 6 0.0 10 0.0 15 0.0 20 0.0 25 0.0 30 1.5 35 4.0 40 8.0 45 11.5 50 15.0 55 17.5 60 19.0 65 20.0 70 20.0 75 20.0 80 20.0arrow_forwardNaming the Alkanes a) Write the IUPAC nomenclature of the compound below b) Draw 4-isopropyl-2,4,5-trimethylheptane, identify the primary, secondary, tertiary, and quaternary carbons. c) Rank pentane, neopentane and isopentane for boiling point. pentane: H3C-CH2-CH2-CH2-CH3 neopentane: CH3 H3C-Ċ-CH3 I CH3 isopentane: CH3 H3C-CH2-CH-CH3arrow_forward
- Which will evaporate faster, 1-Butanol or Pentane? Explain your choice.arrow_forwardUsing the equation below, what is the rate of this reaction if the rate of disappearance of H2 is 0.44 M/sec? H2 + Br2 → 2HBrarrow_forward2Fe3+(aq) + Sn2+(aq) □ 2Fe²+(aq) + Sn 4+ (aq) If the change in Sn²+ concentration is 0.0010M in 38.5 seconds, what is the rate of disappearance of Sn²+?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY