
Concept explainers
Draw the organic products formed when allylic alcoholA is treated with each reagent.
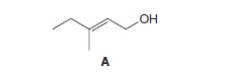
a.
b.
c.
d.
e.
f.
g. [1]
h.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Human Physiology: An Integrated Approach (8th Edition)
Fundamentals Of Thermodynamics
Organic Chemistry
Genetics: From Genes to Genomes
Physics of Everyday Phenomena
- Write the systematic (IUPAC) name for each of the following organic molecules: F structure Br LL Br Br الحمد name ☐ ☐arrow_forwardDraw an appropriate reactant on the left-hand side of this organic reaction. Also, if any additional major products will be formed, add them to the right-hand side of the reaction. + + Х ง C 1. MCPBA Click and drag to start drawing a structure. 2. NaOH, H₂O Explanation Check OI... OH ol OH 18 Ar © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardCalculate the atomic packing factor of quartz, knowing that the number of Si atoms per cm3 is 2.66·1022 and that the atomic radii of silicon and oxygen are, respectively, 0.038 and 0.117 nm.arrow_forward
- 3. a. Use the periodic table to add up the molecular weight of thionyl chloride (SOCl2) and show your work. b. The actual value obtained for the molecular ion on a high resolution mass spectrometer is 117.9041. Explain the discrepancy. c. Show the calculations that correctly result in the exact mass of 117.9041 for SOC₁₂. Use Table 11.2 or Appendix E in your calculations.arrow_forward6. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B₂2+ B22+, B2, C22, B22- and N22+ Molecular Orbital Diagram B2 C22- B22- N22+ Which molecule is paramagnetic?arrow_forwardDon't used hand raitingarrow_forward
- EXERCISES: Complete the following exercises. You must show all work to receive full credit. 1. How many molecular orbitals can be built from the valence shell orbitals in O2? 2. Give the ground state electron configuration (e.g., 02s² 0*2s² П 2p²) for these molecules and deduce its bond order. Ground State Configuration Bond Order H2+ 02 N2arrow_forward7. Draw the Lewis structures and molecular orbital diagrams for CO and NO. What are their bond orders? Are the molecular orbital diagrams similar to their Lewis structures? Explain. CO Lewis Structure NO Lewis Structure CO Bond Order NO Bond Order CO Molecular Orbital Diagram NO Molecular Orbital Diagramarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

