
(a)
Interpretation:
The product of the given reaction should be given.
Concept introduction:
Bromination of Allylic Carbons:

N-bromosuccinimide (NBS) is used for the allylic bromination through radical reaction. bromination of allylicc carbon requires low concentration of bromine and low concentration of hydrobromic acid. If high concentration of bromine and high concentration of hydrobromic acid which leads to the formation of bromination in the double bond.
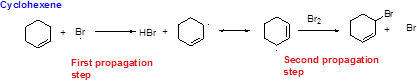
Bromination reaction starts with the homolytic cleavage of
NBS bromine radical removes the allylic hydrogen which forms hydrogen bromide and allylic radical in the first propagation step, the allylic radical is stabilized by the double bond in ring. This allylic radical reaction with bromine molecule and forms allylic bromide in the second propagation step which are shown above.
(a)
Answer to Problem 26P
1-pentene undergoes bromination using N-bromosuccinamide and yields brominated compound A and B which is shown below.

Explanation of Solution
N-bromosuccinimide (NBS) is used for the allylic bromination through radical reaction. Bromination of allylic carbon requires low concentration of bromine and low concentration of hydrobromic acid

Bromination reaction starts with the homolytic cleavage of
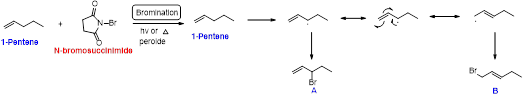
(b)
Interpretation:
The product of the given reaction should be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bromination of Allylic Carbons:

N-bromosuccinimide (NBS) is used for the allylic bromination through radical reaction. bromination of allylic carbon requires low concentration of bromine and low concentration of hydrobromic acid. If high concentration of bromine and high concentration of hydrobromic acid which leads to the formation of bromination in the double bond.

Bromination reaction starts with the homolytic cleavage of
NBS bromine radical removes the allylic hydrogen which forms hydrogen bromide and allylic radical in the first propagation step, the allylic radical is stabilized by the double bond in ring. This allylic radical reaction with bromine molecule forms allylic bromide in the second propagation step which are shown above.
(b)
Answer to Problem 26P
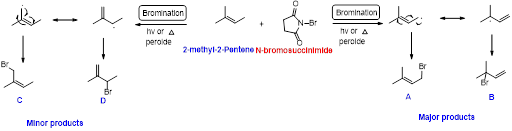
2-methyl-2-pentene undergoes bromination using N-bromo succinamide and yields brominated compound A, B as a major product and C, D as minor product which is shown below.
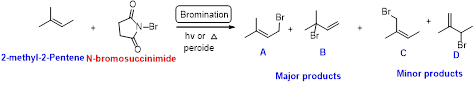
Explanation of Solution
N-bromosuccinimide (NBS) is used for the allylic bromination through radical reaction. bromination of allylicc carbon requires low concentration of bromine and low concentration of hydrobromic acid
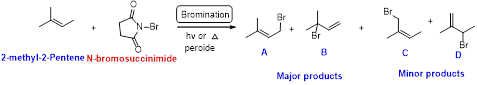
Bromination reaction starts with the homolytic cleavage of
(c)
Interpretation:
The product of the given reaction should be given
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bromination:

2-methyl propane undergoes radical bromination which yields the 2-bromo-2-methylpropane.because bromination will occur where the tertiary radical is present. (bromination reactions are more selective reaction).
Bromination will occur on tertiary radical than the secondary than primary radical, tertiary radical is more stable radical than the other radicals.
(c)
Answer to Problem 26P
3-methyl hexane undergoes radical bromination and yields the 3-bromo-3-methylhexane which is shown below
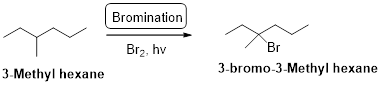
Explanation of Solution
3-methyl hexane undergoes radical bromination and yields the 3-bromo-3-methylhexane which is shown below
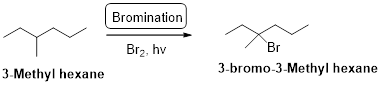
(d)
Interpretation:
The product of the given reaction should be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Chlorination:
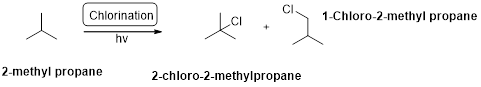
2-methyl propane undergoes radical chlorination and yields the 2-bromo-2-methylpropane and 1-bromo-2-methyl propane.
(d)
Answer to Problem 26P
Cyclohexane undergoes radical chlorination and yields the 1-chloro cyclohexane which is shown below
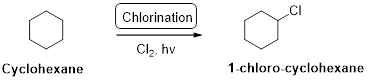
Explanation of Solution
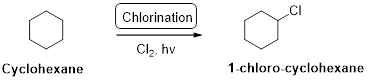
Cyclohexane undergoes radical chlorination, all the carbons in cyclohexane are secondary. Therefore, it yields the 1-chloro cyclohexane which is shown above.
(e)
Interpretation:
The product of the given reaction should be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Chlorination:
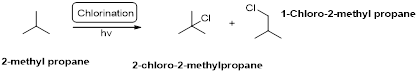
2-methyl propane undergoes radical chlorination and yields the 2-bromo-2-methylpropane and 1-bromo-2-methyl propane.
(e)
Answer to Problem 26P
Cyclopentane has no reaction with chlorine in dichloromethane which is shown below

Explanation of Solution
Cyclopentane has no reaction with chlorine in dichloromethane, because the reaction will not go without light or heat which is shown below

(f)
Interpretation:
The product of the given reaction should be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Chlorination:
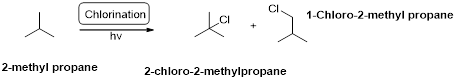
2-methyl propane undergoes radical chlorination and yields the 2-bromo-2-methylpropane and 1-bromo-2-methyl propane.
(f)
Answer to Problem 26P
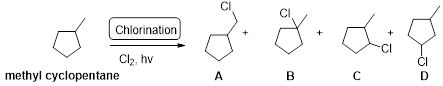
Explanation of Solution
Methyl cyclopentane undergoes radical chlorination, the carbons in cyclopentane are secondary and primary. Therefore, it yields the four types of chlorocyclopentane which is shown below.
Want to see more full solutions like this?
Chapter 12 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
- Assign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forward
- Predict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward
- 个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forwardPredict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forward
- DE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forwardPredict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forwardPredict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





