
Characterize a system at
a. the rates of the forward and reverse reactions
b. the overall composition of the reaction mixture For a general reaction
Interpretation: The given systems at equilibrium are to be characterized. For the given reaction, the required plot is to be shown. The plot that illustrates the rate of forward reaction and rate of reverse reaction is to be sketched.
Concept introduction: Chemical equilibrium is a state of a system in which the rate of forward reaction and that of the backward reaction is equal. It is affected by various factors such as concentration of reactants or products, temperature, pressure.
Answer to Problem 1RQ
Answer
- a) Rate of the forward and reverse reactions are equal at equilibrium.
- b) The overall composition remains constant at equilibrium.
The plot of concentrations of A,B and C versus time is shown in Figure 1.
The plot illustrating the rate of forward reaction and rate of reverse reaction versus time is shown in Figure 2.
Explanation of Solution
Explanation
(I)
(a)
To determine: The characterization of the given system at equilibrium.
The rate of the forward and reverse reactions is equal at equilibrium.
At equilibrium, the concentrations of reactants and products do not change. When equilibrium is attained by a system, equilibrium rate of forward reaction will be equal to rate of backward reaction.
(b)
To determine: The characterization of the given system at equilibrium.
The overall composition remains constant at equilibrium.
The forward and backward reaction at equilibrium proceeds with same rate, hence the concentration of reactants and products do not change. Therefore, the overall composition remains constant at equilibrium.
(II)
To determine: The plot of concentrations of A,B and C versus time and the plot illustrating the rate of forward reaction and rate of reverse reaction versus time.
The plot of concentrations of A, B and C versus time is shown in Figure 1.
The given reaction is,
If one starts with only reactants present, then the plot of concentrations of A,B and C versus time will be like,
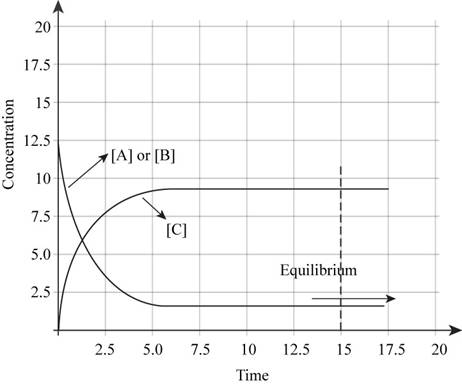
Figure 1
In the beginning of the reaction, the concentration of reactants is more. As the time proceeds, the concentration of reactants decreases and the concentration of products increases. At equilibrium, there is no change in the concentrations of reactants and products.
The plot illustrating the rate of forward reaction and rate of reverse reaction versus time is shown in Figure 2.
In the beginning of the reaction, the concentration of reactants is more. Therefore, rate of forward reaction is high. But as the reaction proceeds further, the reactants are consumed to form products. Hence rate of forward reaction decreases and rate of backward reaction increases. At equilibrium, both the rates are equal. Therefore, plot illustrating the rate of forward reaction and rate of reverse reaction versus time will be like,
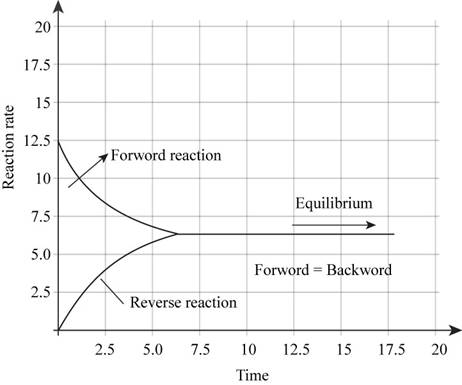
Figure 2
Conclusion
- a) Rate of the forward and reverse reactions are equal at equilibrium.
- b) The overall composition remains constant at equilibrium.
The plot of concentrations of A, B and C versus time is shown in Figure 1.
The plot illustrating the rate of forward reaction and rate of reverse reaction versus time is shown in Figure 2
Want to see more full solutions like this?
Chapter 12 Solutions
Bundle: Chemistry: An Atoms First Approach, 2nd, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
- Using Luther's rule, determine the reference potentials of the electrodes corresponding to the low stability systems Co³+/Co and Cr²+/Cr from the data in the table. Electrodo ΕΝ Co²+/Co Co3+/Co²+ -0,28 +1,808 Cr³+ / Cr -0,508 Cr3+ / Cr²+ -0,41arrow_forwardThe molecule PYRIDINE, 6tt electrons and is there pore aromuntre and is Assigned the Following structure contenus Since aromatk moleculey undergo electrophilic allomatic substitution, Pyridine should undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this roaction Based upon the reaction the reaction mechanism determine which of these producty would be the major Product of the hegetionarrow_forwardUsing Benzene as starting materia Show how each of the Following molecules could Ve synthesked 9. CHI d. 10450 b 0 -50311 ८ City -5034 1-0-650 e NO2arrow_forward
- BA HBr of the fol 1)=MgCI 2) H₂O major NaOEt Ts Cl Py (pyridine) 1) 03 2) Me2S 1arrow_forward4. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a) NHBoc ⚫OBn HO. H3C CO2CH3 -OBn H3C H3C. H3C. NHBOC CI CO2CH3arrow_forwardDraw structures of the following compounds and identify their role: mCPBA (MCPBA) DMS Py 9-BBN LAH Sia₂BH TsCI PCC t-BuOK LDA MeLi n-BuLi DMSO DMF Sodium Borohydride Lithium DiisopropylAmide 2arrow_forward
- Using Luther's rule, calculate the reference potential of the Hg2+/Hg redox electrode. DATA: Electrode potentials E° = 0,854 V y E 0,788 V Hg2+/Hg 2+ Hg2/Hgarrow_forward1) NaNH2 (excess) 1) NaNH2 CI CI 2) H₂O 2) Mel 1) 03 2) (CH3)2S Na NH3 (liquid) 1arrow_forwardCI 1) n-BuLi 2) 1) 03 HH T&Cl 2) H₂O 2arrow_forward
- Help with a!arrow_forwardFor the following compound: HO -H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: H-O: H-OH H-O HH H-OO Mechanism B: H-Q Mechanism C: Θ OH H-O: Mechanism D: H-O H- H-OO C H-OO H- H- H-OO HH OH -H - HON H :OH H-Harrow_forwardidentify the product (or multiple products) for each of the following reactions: CI 1) NaNH2 (excess) ठ Cl 2) H₂O Hz H₂SO₂, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) 1)9-BBN 2) H₂O, NaOH ? Br H A B C afó gó H OA B O c OD E OF D E F H H Na, NHarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





