
Chemistry
3rd Edition
ISBN: 9780073402734
Author: Julia Burdge
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 14QP
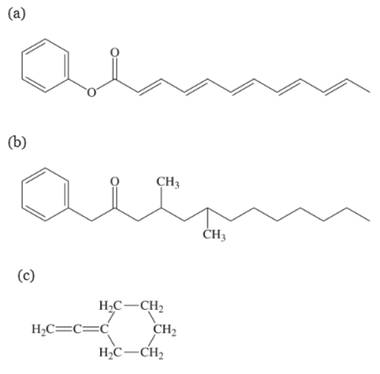
Would each of these molecules be likely to form a liquid crystal? If not, why not?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(EXM 2, PRBLM 3) Here is this problem, can you explain it to me and show how its done. Thank you I need to see the work for like prbl solving.
can someone draw out the reaction mechanism for this reaction showing all bonds, intermediates and side products
Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided below
What would be the best choices for the missing reagents 1 and 3 in this synthesis?
1. PPh3
3
2. n-BuLi
• Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like.
• Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is.
• Note: if one of your reagents needs to contain a halogen, use bromine.
Click and drag to start drawing a structure.
Chapter 12 Solutions
Chemistry
Ch. 12.1 - Practice ProblemATTEMPT Saran Wrap, the original...Ch. 12.1 - Prob. 1PPBCh. 12.1 - Practice ProblemCONCEPTUALIZE Which diagram best...Ch. 12.1 - Prob. 1CPCh. 12.1 - Prob. 2CPCh. 12.2 - Prob. 1PPACh. 12.2 - Practice ProblemBUILD Kodel is a polymer used to...Ch. 12.2 - Prob. 1PPCCh. 12.3 - Prob. 1PPACh. 12.3 - Prob. 1PPB
Ch. 12.3 - Prob. 1PPCCh. 12.3 - Prob. 1CPCh. 12.3 - Prob. 2CPCh. 12.4 - Prob. 1PPACh. 12.4 - Prob. 1PPBCh. 12.4 - Prob. 1PPCCh. 12.5 - Prob. 1PPACh. 12.5 - Prob. 1PPBCh. 12.5 - Prob. 1PPCCh. 12.6 - Prob. 1CPCh. 12.6 - Prob. 2CPCh. 12 - 12.1 Bakelite, the first commercially produced...Ch. 12 - Prob. 2QPCh. 12 - Prob. 3QPCh. 12 - Prob. 4QPCh. 12 - Prob. 5QPCh. 12 - Prob. 6QPCh. 12 - Prob. 7QPCh. 12 - Prob. 8QPCh. 12 - Prob. 9QPCh. 12 - Prob. 10QPCh. 12 - Bakelite. described in Review Question 12.1, is...Ch. 12 - Is a normal liquid isotropic or anisotropic? How...Ch. 12 - Prob. 13QPCh. 12 - 12.14 Would each of these molecules be likely to...Ch. 12 - Prob. 15QPCh. 12 - 12.16 Would an ionic compound form a liquid...Ch. 12 - Prob. 17QPCh. 12 - Prob. 18QPCh. 12 - Prob. 19QPCh. 12 - 12.20 What are some advantages and disadvantages...Ch. 12 - Prob. 21QPCh. 12 - 12.22 How does an measure the peak and valley...Ch. 12 - Name four allotropic forms of carbon.Ch. 12 - Prob. 24QPCh. 12 - Prob. 25QPCh. 12 - Prob. 26QPCh. 12 - 12.27 What type of intermolecular forces holds the...Ch. 12 - Prob. 28QPCh. 12 - Prob. 29QPCh. 12 - Prob. 30QPCh. 12 - Prob. 31QPCh. 12 - Prob. 32QPCh. 12 - Prob. 33QPCh. 12 - Prob. 34QPCh. 12 - Prob. 35QPCh. 12 - Prob. 36QPCh. 12 - What types of bonding (covalent, ionic, network,...Ch. 12 - Draw representations of isotactic, syndiotactic....Ch. 12 - Prob. 39QPCh. 12 - Draw representations of block copolymers and graft...Ch. 12 - Prob. 41APCh. 12 - 12.42 Would the compound shown form a liquid...Ch. 12 - Prob. 43APCh. 12 - Prob. 44APCh. 12 - Fluoride ion is commonly used in drinking water...Ch. 12 - Prob. 1SEPPCh. 12 - Prob. 2SEPPCh. 12 - Prob. 3SEPPCh. 12 - Prob. 4SEPP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the missing organic reactants in the following reaction: X + Y H+ two steps Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Х :arrow_forwardDraw the mechanism of friedel-crafts acylation using acetyl chloride of m-Xylenearrow_forwardI need help naming these in IUPACarrow_forward
- H R Part: 1/2 :CI: is a/an electrophile Part 2 of 2 Draw the skeletal structure of the product(s) for the Lewis acid-base reaction. Include lone pairs and formal charges (if applicable) on the structures. 4-7: H ö- H Skip Part Check X :C1: $ % L Fi Click and drag to start drawing a structure. MacBook Pro & ㅁ x G 0: P Add or increase positive formal cha Save For Later Submit ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forwardDraw the friedel-crafts acylation mechanism of m-Xylenearrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- 1. Base on this experimental results, how do you know that the product which you are turning in is methyl 3-nitrobenzoate(meta substituted product ) rather than either of the other two products? 2. What observation suggests that at least a small amount of one or both of the other two isomers are in the mother liquor?arrow_forwardExplain Huckel's rule.arrow_forwardhere is my question can u help me please!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=HCWwRh5CXYU;License: Standard YouTube License, CC-BY